(C) 2011 I.A. Gavrilov-Zimin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
New cytogenetic data are reported for 17 species from 15 genera of the families Pseudococcidae, Eriococcidae, Kermesidae, and Coccidae. Twelve species and 6 genera (Peliococcopsis Borchsenius, 1948, Heterococcopsis Borchsenius, 1948, Heliococcus Šulc, 1912, Trabutina Marchal, 1904, Lecanopsis Targioni Tozzetti, 1868, and Anapulvinaria Borchsenius, 1952) were studied cytogenetically for the first time. The taxonomic problems in the genera Trionymus Berg, 1899, Acanthopulvinaria Borchsenius, 1952 and Rhizopulvinaria Borchsenius, 1952 are discussed based on karyotype characters. Two chromosomal forms (cryptic species) of Acanthopulvinaria orientalis(Nasonov, 1908), 2n=18 and 2n=16 were discovered.
scale insects, karyotypes, chromosome numbers, karyosystematics
In June 2009 the author and Dr. Mehmet Bora Kaydan made several joint collecting trips in Eastern Anatolia (Turkey). Part of the material collected during these trips, plus some other material collected by M.B. Kaydan without me in 2009, proved to be suitable for cytogenetic studies. Turkey in general and especially Eastern Anatolia have an exceptionally rich scale insect fauna (
Until now, Palaearctic scale insects were studied cytogenetically rather fragmentarily and significantly more poorly than tropical and subtropical species (
The unique genetic systems of scale insects (XX-X0, n-2n (Haplo-diploidy), Hermaphroditism, 2n-2n, Lecanoid, Comstockioid, Diaspidoid, obligate Thelytoky) have been reviewed many times in special papers (
Some scale insects (in particular, some of those listed below) are characterized by a unique individual development that is similar to a double fertilization in angiosperms. In this case each embryo develops from two different cells. One of those is a normal diploid zygote that gives rise to the majority of tissues. The other cell is a polyploid secondary zygote that results from the fusion of a cleavage nucleus with the first or second polar bodies. The secondary zygote gives rise to the polyploid bacteriome (or mycetome). Each cell of the bacteriome (or mycetome) thus includes one haploid set of paternal chromosomes and several maternal sets (
All material for this study was collected in 2009 in Eastern Anatolia (Turkey). The detailed collecting data are listed below, separately for each species in order to avoid the double citations of taxonomic names and for more comfortable using of the paper.
The chromosomal plates were made as previously described (
All material is deposited at the Zoological Institute, Russian Academy of Sciences, St. Petersburg.
Results and discussion Family PseudococcidaePuto superbus (Leonardi, 1907)
Figs 1–3
Material. K 607, Igdir-Digor road, 40°10'451"N, 43°40'389"E, on steams of grass, 04.06.2009, M.B. Kaydan & I. Gavrilov.
Embryos from female body. 2n=16 +XX (♀), 2n= 16 +X0 (♂).
Hitherto, only American species of the genus Puto Signoret, 1876 were studied cytogenetically (Hughes-Schrader 1944, Brown and Clevelend 1966). Puto superbus is the first studied species of the genus from the Palaearctic fauna; it also has an ancient XX/X0 genetic system (as 5 other studied species of the genus), but demonstrates a different chromosome number (2n=18/17) in contrast to 2n=14/13, 16/15, 20/19 in American species.
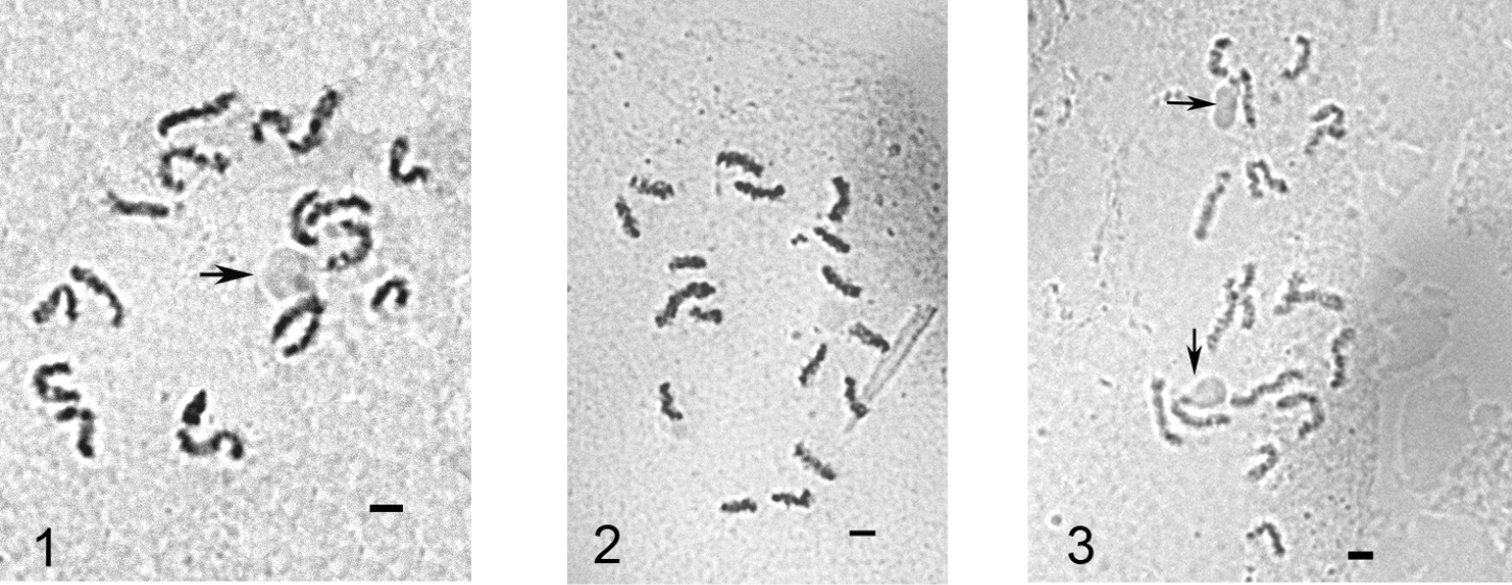
Mitotic chromosomes of Puto superbus. 1 cell of female embryo, 2n=18 2, 3 cells of male embryo, 2n=17. The chromosomes with NORs are arrowed. Bar = 10 µm.
Figures 1 & 3 illustrate nucleoli localized at the ends of the middle-sized chromosomes. The localizations of NORs in scale insects were discussed earlier by
Phenacoccus Cockerell, 1893
Hitherto, 16 species of the large and widely distributed genus Phenacoccus have been studied by different authors (see the review of
Sharing the same chromosomal number Phenacoccus spp. demonstrate, however, significant variation of chromosomal lengths in their karyotypes. This variation in combination with the data on differential staining of Phenacoccus spp. chromosomes will probably provide the basis for further karyotaxonomic studies of the genus.
Phenacoccus specificus Matesova, 1960
Fig. 4
Material. K 603 (4472), Kars - Kagizman road, 40°16'351"N, 42°52'275"E, 1761 m alt., on roots of Thymus sp., 04.06.2009, M.B. Kaydan & I. Gavrilov.
Embryos from female body. 2n=10.
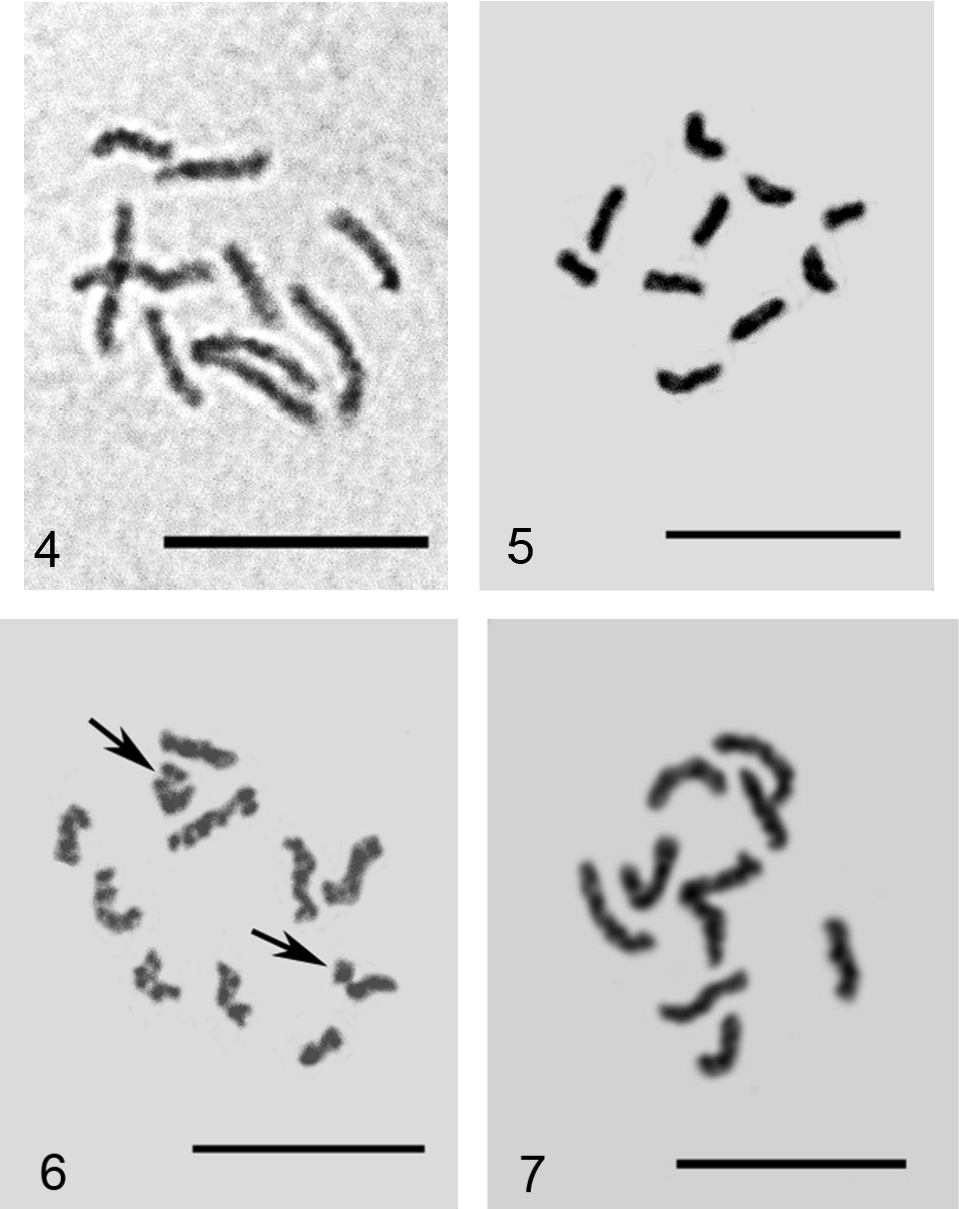
Embryonic cells of Phenacoccus spp. 4 Phenacoccus specificus, 2n=10 5, 6 Phenacoccus phenacoccoides: 5 2n=10, 6 2n=10+Bs, additional chromosomal elements arrowed 7 Phenacoccus tergrigorianae, 2n=10. Bar = 10 µm.
Phenacoccus phenacoccoides (Kiritshenko, 1932)
Figs 5–6
Material. K 612 (4483), Kars-Kagizman road, 40°12'011"N, 43°02'827"E, 1273 m alt., under the leaf sheaths of grass, 04.06.2009, M.B. Kaydan & I. Gavrilov.
Embryos from female body. 2n=10, 2n=10 + Bs, Lecanoid heterochromatinization.
The studied population of Phenacoccus phenacoccoides demonstrates variation from 0 to 2 additional chromosomal elements (B-chromosomes) between embryonic cells like as seen in a population from the Voronezh region (central part of European Russia) studied earlier by me (
Phenacoccus tergrigorianae Borchsenius, 1956
Fig. 7
Material. K 693, Van-Hakkari road, 37°32'340"N, 43°43'173"E, on roots of undetermined Asteraceae, 02.09.2009, M.B. Kaydan. K 689, the same data, but on Sorghum halepense.
2n=10, Lecanoid heterochromatinization.
Peliococcopsis priesneri (Laing, 1936)
Figs 8–9
Material. K 601, Agri-Dogubeyazid-Ishakpasa, 39°31'905"N, 44°07'100"E, 2059 m alt., under the leaf sheaths of Cynodon dactylon, 03.06.2009, M.B. Kaydan & I. Gavrilov.
Embryos from female body. 2n=10, Lecanoid heterochromatinization.
It is the first species of the genus Peliococcopsis Borchsenius, 1948 studied cytogenetically.
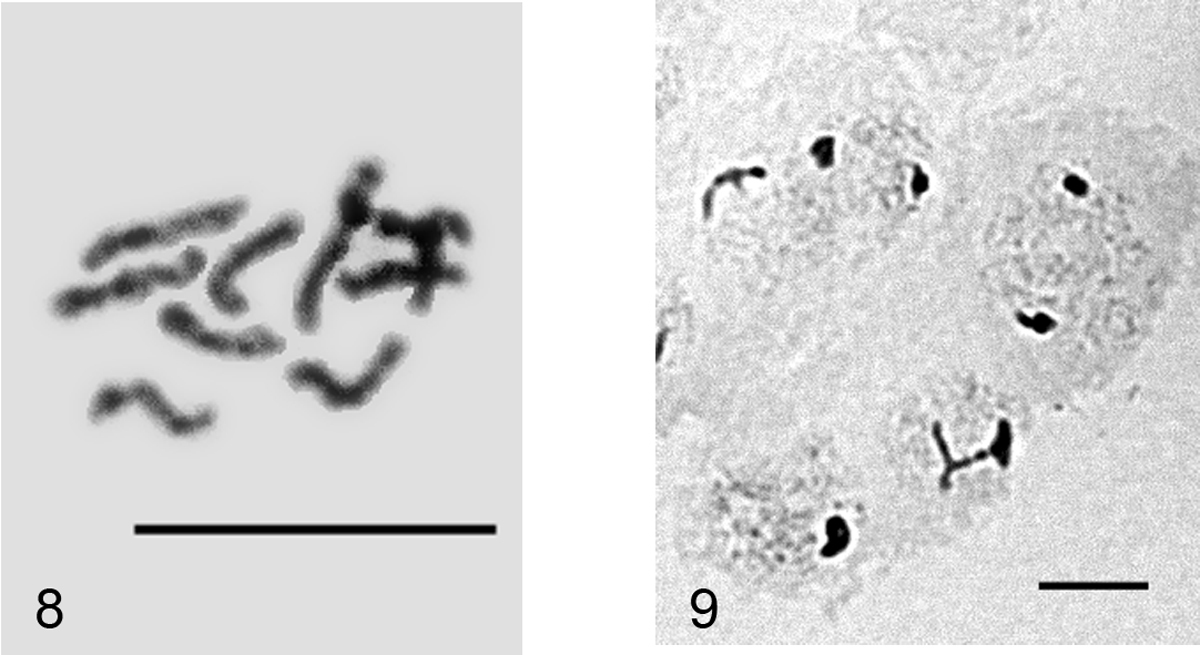
Peliococcopsis priesneri. 8 mitotic chromosomes, 2n=10 9 male embryonic cells at interphase stage with one haploid set heterochomatinized. Bar = 10 µm.
Heterococcopsis opertus Borchsenius, 1949
Fig. 10
Material. 4530, Eastern Anatolia without concrete location, 2009, M.B. Kaydan.
Embryos from female body. 2n=10.
It is the first species of the genus Heterococcopsis Borchsenius, 1948 studied cytogenetically.
Heterococcopsis opertus, embryonic cell, 2n=10. Bar = 10 µm.
Heliococcus sulci Goux, 1934
Fig. 11
Material. K677, Hatay-Erzin, 08.09.2009, M.B. Kaydan.
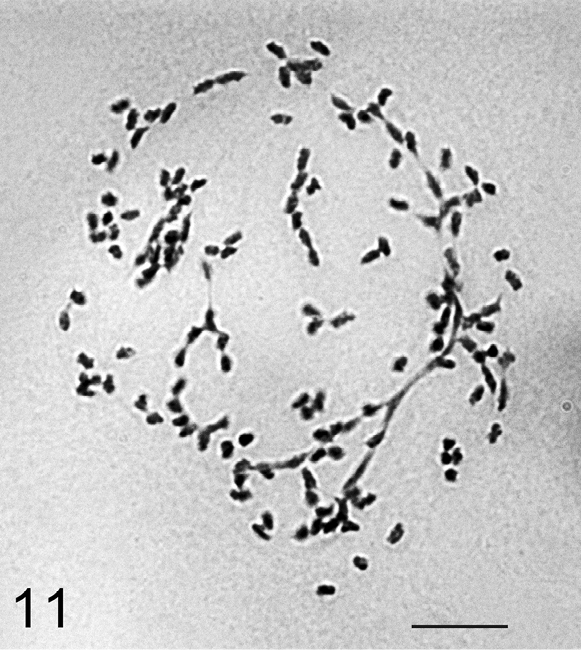
Only one female was available for cytogenetic studies and the specimen did not provide cells with chromosome plates suitable for karyotype study. However, some polyploid cells of the mycetome with about 140 chromosomes and numerous agglutinations were observed. In view of the absence till now of any cytogenetic data on the large and very important for phylogenetic reconstructions genus Heliococcus Šulc, 1912 I am presenting here the first photograph of Heliococcus chromosomes (Fig. 11). It appears that there is no significant size difference between chromosomes.
Heliococcus sulci, the cell of mycetome, about 140 chromosomes with numerous agglutinations. Bar = 10 µm
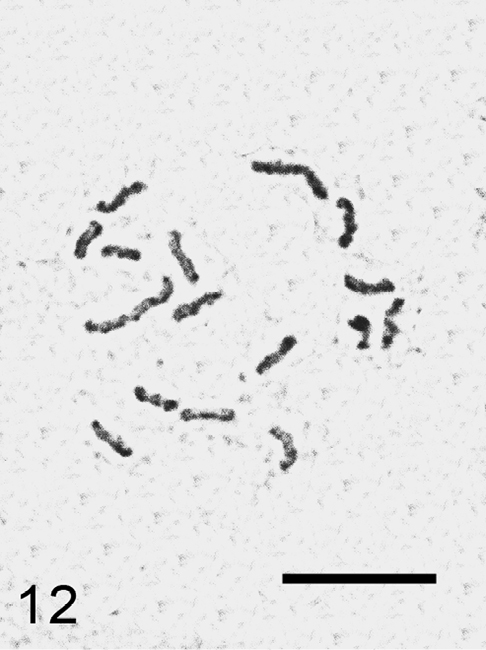
Trabutina crassispinosa Borchsenius, 1941
Fig. 12
Material. K 605, Igdir-Digor road, Kars border, 40°07'278"N, 43°37'708"E, on branch of Tamarix sp., 04.06.2009, M.B. Kaydan & I. Gavrilov.
Embryos from the ovisacs. 2n=16.
It is the first species of the genus Trabutina Marchal, 1904 studied cytogenetically.
Trabutina crassispinosa, cell of embryo, 2n=16. Bar = 10 µm.
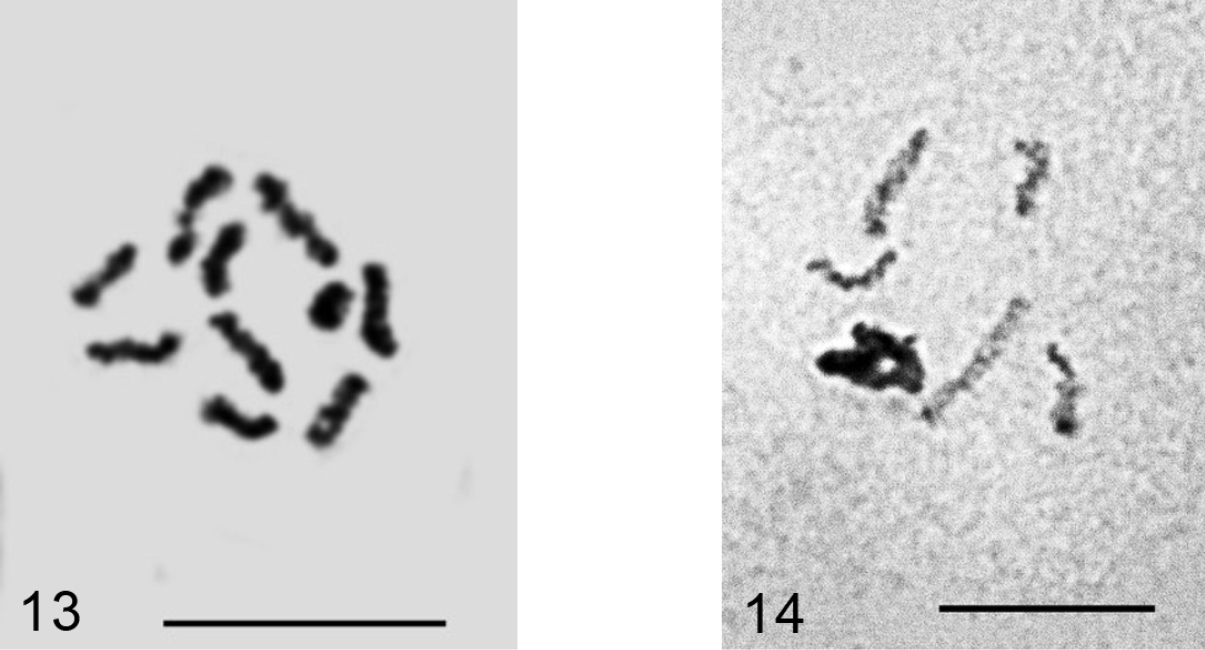
Planococcus vovae (Nasonov, 1908)
Figs 13–14
Material. K 686, Şanliurfa, on Cupressus sp., 09.09.2009, M.B. Kaydan.
2n=10, Lecanoid heterochromatinization. The studied population deviates morphologically from the usual Planococcus vovae having 2 circuli in contrast to 1 (or, exceptionally, none) in huge material from different regions of the Palaearctic (
Planococcus vovae 13 mitotic chromosomes, 2n=10 14 male embryonic cell with one haploid set heterochomatinized. Bar = 10 µm.
Dysmicoccus multivorus (Kiritshenko, 1936)
Figs 15–16
Material. K 685, Van-Akdamar, on undetermined Apiaceae, 09.06.2009, M.B. Kaydan & I. Gavrilov.
2n=10+1-2 B. All previously studied populations of this species (
Dysmicoccus multivorus. 15 embryonic cell with 2n=10 + B, additional chromosomal element arrowed 16 cell of mycetome, 7x=35. Bar = 10 µm
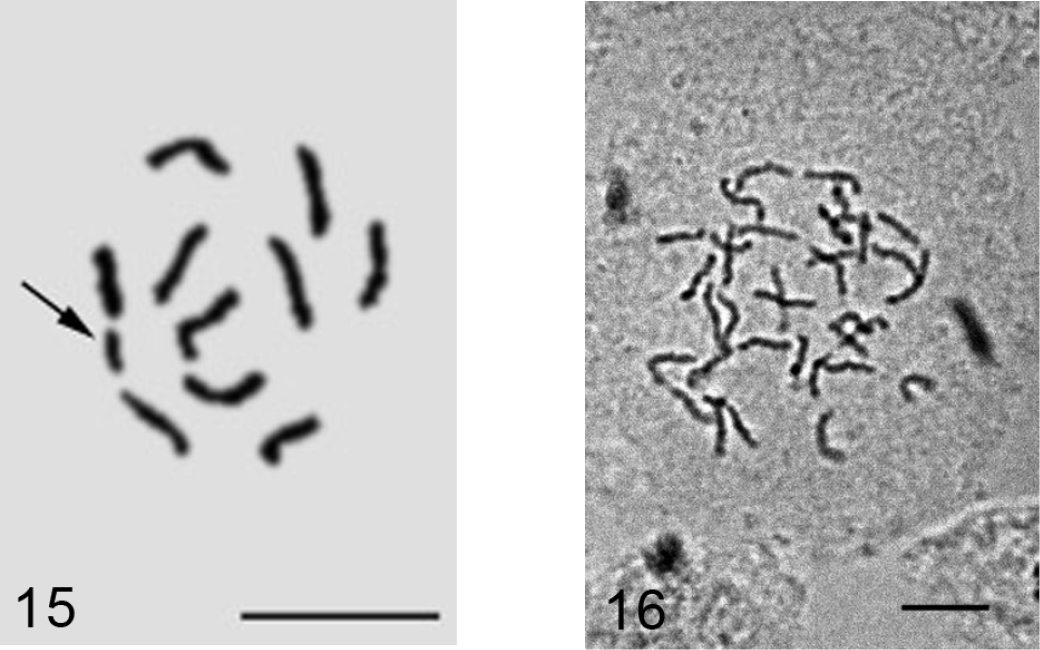
Trionymus artemisiarum (Borchsenius, 1949)
Fig. 17
Material. K 680 (4536), Ağri-Patnos-Adilcevaz road-Aktepe, on Achillea sp., 10.06.2009, M.B. Kaydan.
Embryos from female body. However, oviposition takes place during earlier stages of embryonic development, before gastrulation.
2n=10, Lecanoid heterochromatinization.
The species demonstrates another new example of karyotaxonomic rule discovered by the author (
Trionymus artemisiarum, 2n=10, cell of male embryo, one haploid set (arrowed) is heterochomatinized. Bar = 10 µm.
Acanthococcus lactucae Borchsenius, 1949
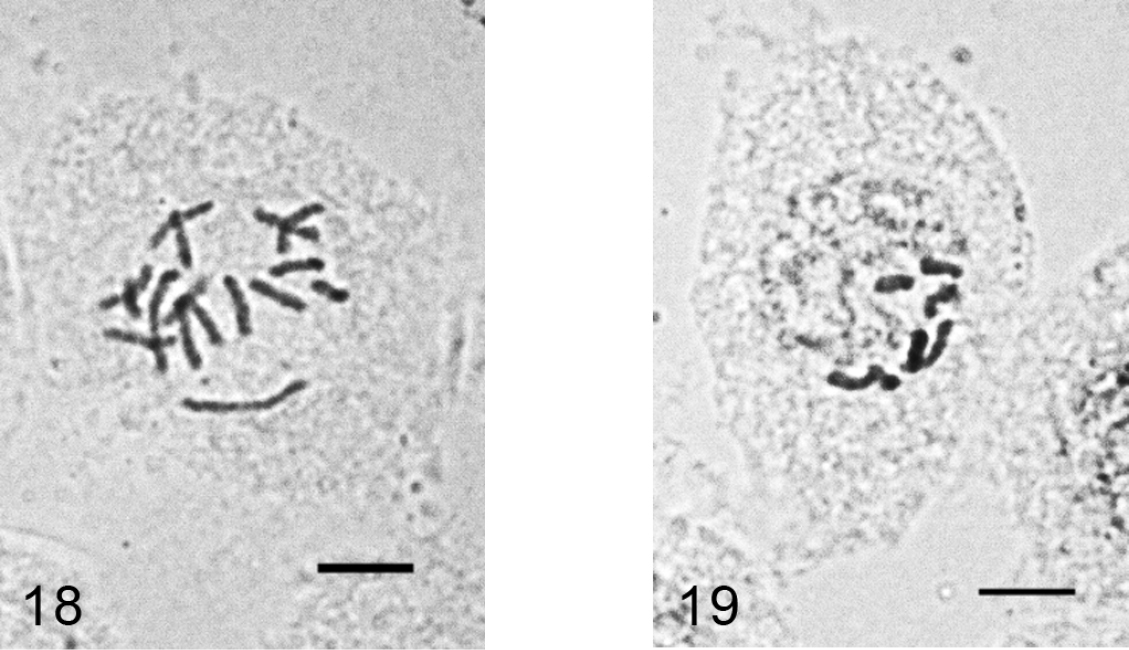
Figs 18–19
Material. K 675 (4555), Hakkari-Çukurca road, on Cichorium sp., 01.09.2009, M.B. Kaydan.
Embryos from female body.
2n=16, heterochromatinization, presumably Comstockioid. The same characteristics have been previously detected in two other species of this genus (
Acanthococcus lactucae. 18 mitotic chromosomes in female embryo, 2n=10. 19 - male embryonic cell in interphase with one haploid set heterochomatinized. Bar = 10 µm.
Kermes roboris (Fourcroy, 1785)
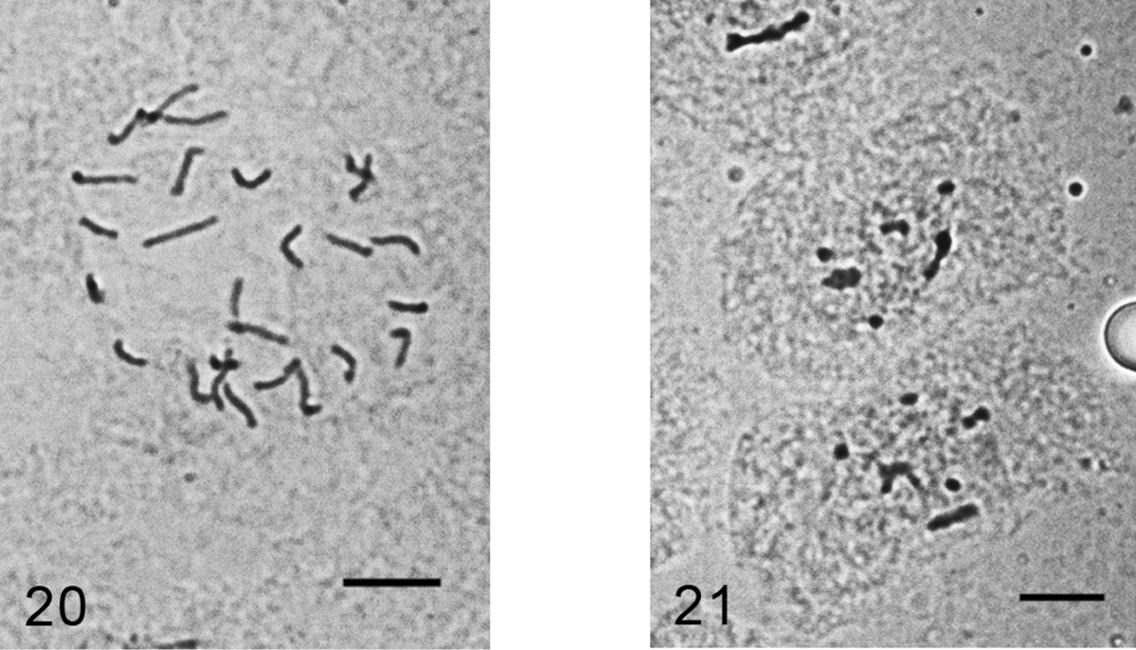
Figs 20–21
Material. K 636, Tatvan-Güroymak road, 38°33'187"N, 42°05'851"E, 1570 m alt., on twigs of Quercus sp., 10.06. 2009, I. Gavrilov.
Embryos from female body. 2n=26, heterochromatinization, presumably of the Comstockioid type. It is the second studied species of the genus Kermes Linnaeus, 1758 and of the whole family Kermesidae. The first studied species, Kermes quercus (Linnaeus, 1758), also has 2n=26 and presumable Comstockioid heterochromatinization (
Kermes roboris. 20 mitotic chromosomes in female embryo, 2n=26 21 male embryonic cell in interphase with one haploid set heterochomatinized. Bar = 10 µm.
Lecanopsis turcica (Bodenheimer, 1951)
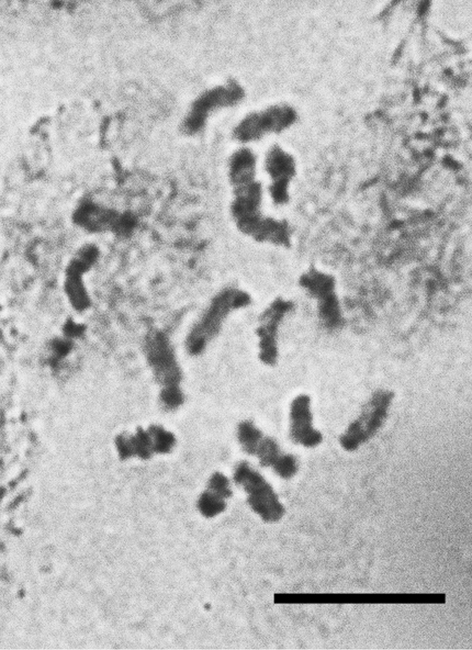
Figs 22
Material. K 592, Dogubeyazit – Igdir road, 39°46'51"N, 44°08'584"E, 1552 m alt., on roots of undetermined Poaceae, 03.06.09, I. Gavrilov.
Embryos from female body. 2n=18, heterochomatinization of an unidentified type. It is the first species of comparatively large Palaearctic genus Lecanopsis Targioni Tozzetti, 1868 studied cytogenetically.
Lecanopsis turcica, cell of female embryo, 2n=18. Bar = 10 µm.
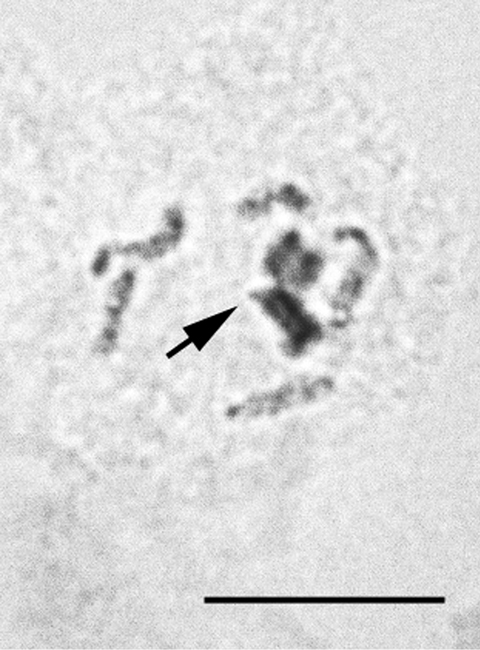
Acanthopulvinaria orientalis (Nasonov, 1908)
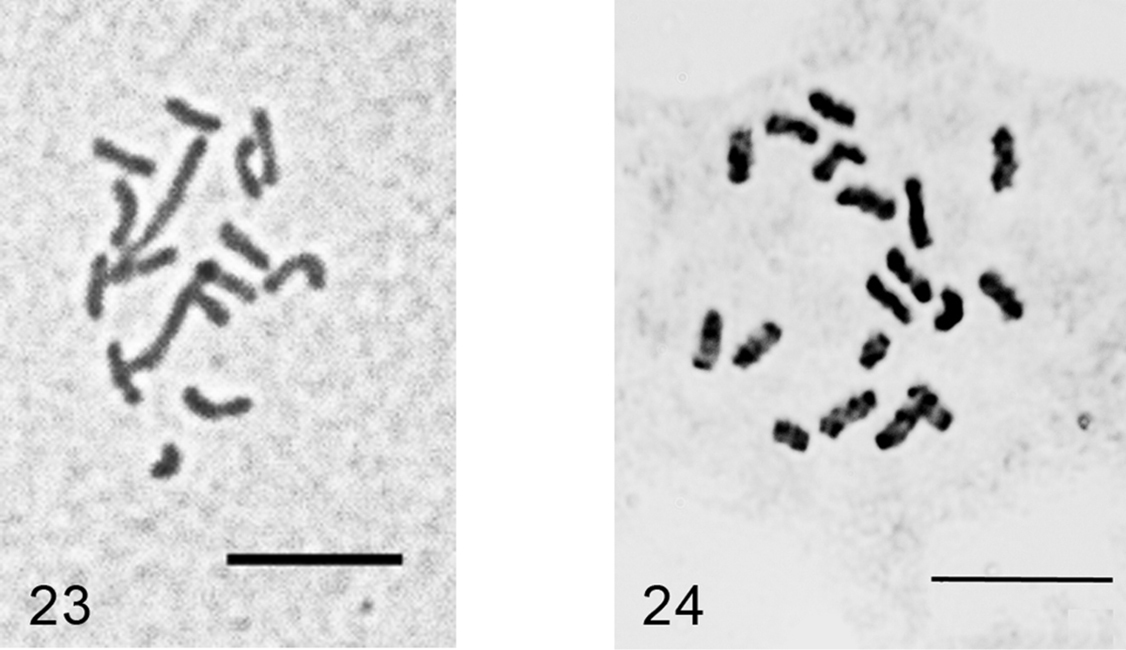
Figs 23–24
Material. K 631 (4502), Van Gurpinar road, on steams of Artemisia sp., M.B. Kaydan & I. Gavrilov.
Embryos from ovisacs. 2n=16, heterochromatinization of an unidentified type.
These new data revealed that Acanthopulvinaria orientalis, earlier studied from Astrakhan only (Russian coast of the Caspian Sea) (
Since 2n=16 and 2n=18 chromosomal sets obviously cannot produce fertile hybrid progeny due to meiotic abnormalities they should be treated as two separate species. However, for a final taxonomic decision it is necessary to study more populations from different parts of Acanthopulvinaria orientalis geographic area.
Acanthopulvinaria orientalis, cells of female embryo 23 Turkey, 2n=16 24 Astrakhan (Russia), 2n=18, after
Rhizopulvinaria artemisiae (Signoret, 1873)
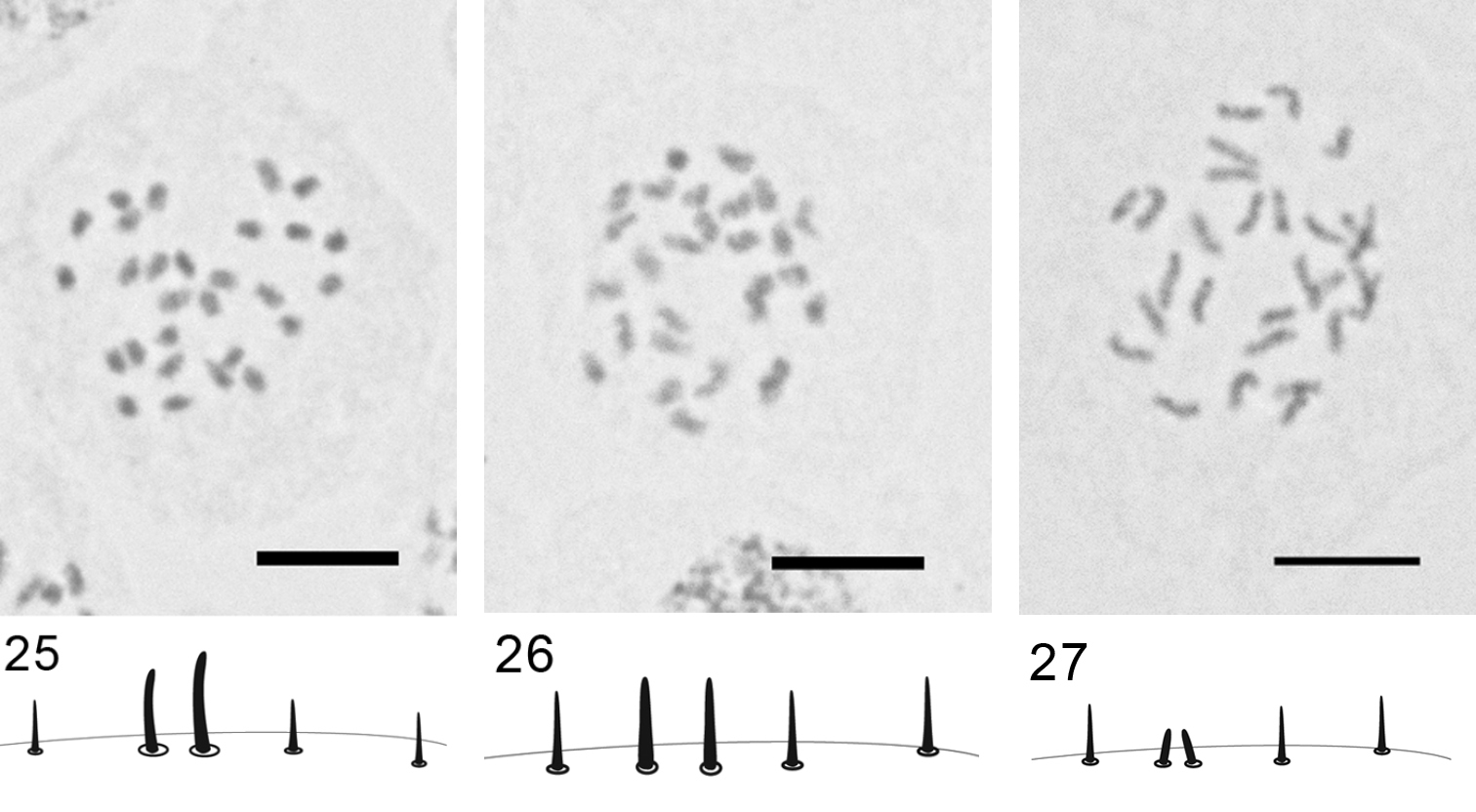
Figs 25–27
Material. K 595 (4462), Çaldiran-Dogubeyazit road, 39°11'71"N, 44°00'784"E, on Acantholimon sp., 03.06.09, M.B. Kaydan & I. Gavrilov. K 598 (4467), Agri-Dogubeyazit-Ishakpaşa Palace, 39°31'905"N, 44°07'100"E, 2059 m alt., on Artemisia sp., 03.06.09, M.B. Kaydan & I. Gavrilov. K 610 (4481), Kars-Kagizman road, 40°12'011"N, 43°02'827"E, 1273 m alt., on Artemisia sp., 04.06.09, M.B. Kaydan & I. Gavrilov.
Embryos from female body. 2n=28, male embryos with heterochromatinization are absent.
Three Turkish populations studied here show the same karyotype with 28 approximately equal in size chromosomes as in a previously studied population from Astrakhan (
Rhizopulvinaria artemisiae,2n=28, embryonic cells and marginal setae 25 population K 595 26 population K 598 27 population K 610. Bar = 10 µm
Anapulvinaria pistaciae (Bodenheimer, 1926)
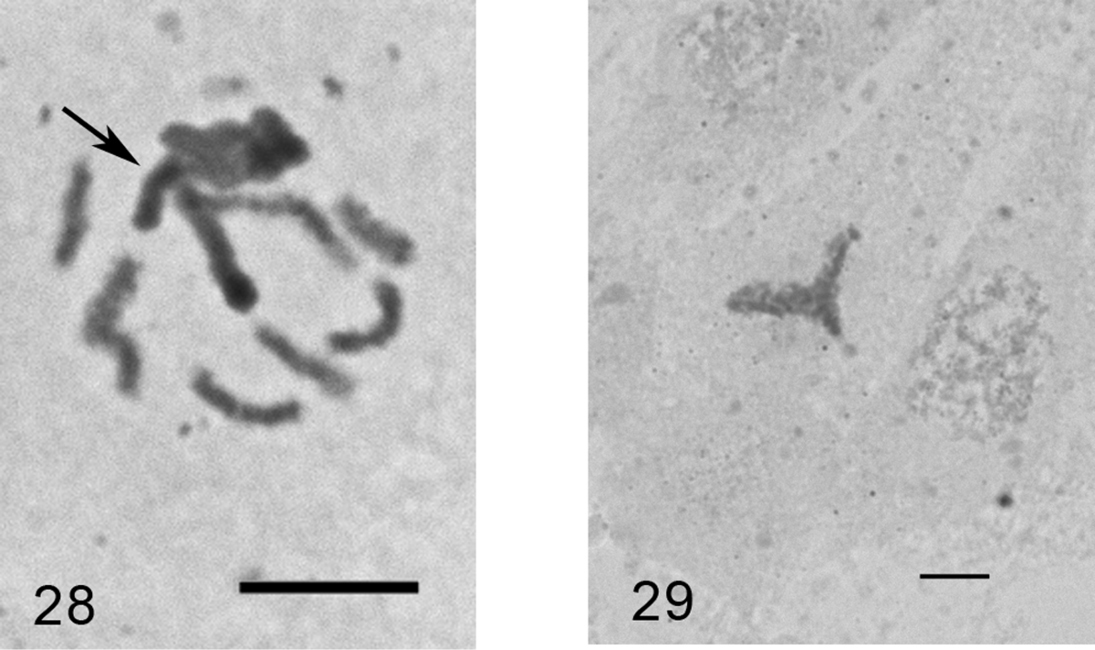
Figs 28–29
Material. K 647, Bitlis, ruins of ancient fortress, on twig of Pistacia sp., 10.06.2009, M.B. Kaydan & I. Gavrilov.
Eggs from female body. The laid eggs are of two colors: white and brown.
2n=16?, heterochromatinization.
The monotypic genus Anapulvinaria Borchsenius, 1952 was studied here cytogenetically for the first time. Unfortunately, the only female with ovisac was collected and analyzed; the embryos (more than 100 were squashed) demonstrated numerous tripolar mitotic divisions. According to this abnormality and also due to a small number of chromosomal plates suitable for karyotype analysis (2 cells of female embryo and 3 cells of male embryo in total) I am giving the chromosomal number with small doubt. Some embryos contained polyploid cells with about 50 chromosomes.
Anapulvinaria pistaciae, embryonic cells 28 cell of male embryo with one haploid set heterochromatinized (arrowed) and 8 euchomatic chromosomes 29 tripolar mitosis. Bar = 10 µm.
I am very grateful to Dr. M. Bora Kaydan for the possibility to visit a lot of interesting natural localities of Eastern Anatolia, providing the interesting material and for reviewing of the manuscript.
I thank very much Dr. Demian Takumasa Kondo for his scientific and linguistic corrections and remarks.
This work was supported by the grant of the President of the Russian Federation (MK-6075.2010.4), RFBR 11-04-00734-a and by special grant of the St. Petersburg Government.
The collections of the Zoological Institute RAS were supported by the ministry of science and education of the Russian Federation.