(C) 2012 Diogo Teruo Hashimoto Tatiana Aparecida Voltolin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The aim of this study was to characterize cytogenetically one population of the fish Moenkhausia sanctaefilomenae (Steindachner, 1907), with emphasis on the analysis of B chromosomes. The nucleolar activity in the B microchromosomes was characterized, and an analysis of mitotic instability of these microchromosomes was accomplished. The results showed a diploid chromosome number of 50 chromosomes. In all individuals, we observed the presence of B microchromosomes with intra- and inter-individual variability. The analysis of the nucleolus organizing regions (NORs) by silver nitrate staining demonstrated multiple NORs. We observed active sites of ribosomal DNA in the B microchromosomes, with a frequency of 20% in the analyzed cells, which shows gene activity in these chromosomal elements. The analysis of constitutive heterochromatin patterns showed that the B microchromosomes are heterochromatic or euchromatic, which demonstrates differentiation of DNA composition between these genomic elements. The calculation of the mitotic instability index implied that B chromosomes in this species might be in a final stage of instability.
fish cytogenetic, NOR expression, supernumerary chromosomes, mitotic instability
Moenkhausia Eigenmann, 1903 is considered as incertae sedis in Characidae and contains 65 valid species widely distributed in the Neotropical river basins (
Chromosome studies in the genus Moenkhausia are still restricted and cytogenetic data are available only for six species (
B chromosome includes a variety of extra chromosomes that display conspicuous heterogeneity in their nature, behavior, and evolutionary dynamics. This definition highlights some of the most universal properties of B chromosomes: their dispensability (that is, they are not necessary for the host to complete a normal life cycle); their origin from chromosomes (either from within the same species or from other species); and their remarkable differentiation relative to A chromosomes, with which they do not recombine (
B chromosomes are widely distributed among eukaryotes and their occurrence has been reported in 10 species of the fungi, nearly 1.300 plants (more than 1.400 when different ploidy levels of the same species are considered separately), and over 500 animals (
In species of Moenkhausia, B chromosomes were documented for Moenkhausia sanctaefilomenae and Moenkhausia intermedia Eigenmann, 1908 (
Another interesting characteristic observed in the B microchromosomes of Moenkhausia sanctaefilomenae is the polymorphism revealed by C-banding. Through this method, these microchromosomes can be characterized in different classes according to the pattern of constitutive heterochromatin; they can be partially and totally heterochromatic, and euchromatic (
In the present study, we carried out cytogenetic analyses in one particular population of the fish Moenkhausia sanctaefilomenae focusing on two special features concerning the B microchromosomes: the occurrence of nucleolar activity in the B chromosome of this species and a study about the maintenance of microchromosomes in this population through the calculation of the mitotic instability index (MI).
Material and methodsThe cytogenetic analyses were carried out in chromosomal preparations obtained from 15 specimens (8 males and 7 females) of Moenkhausia sanctaefilomenae. The individuals were collected from a population of the Batalha River (22°7.02'S, 49°16.01'W), belonging to Tietê River basin, São Paulo State, southeastern Brazil. The voucher specimens were identified and stored in the fish collection of the Laboratório de Genética de Peixes, UNESP, Bauru, SP, Brazil.
Before sacrifice, the animals were inoculated with yeast cell suspension to increase the number of metaphase cells (
The index to quantify the mitotic instability of B chromosome, MI, which was calculated as the sum of the absolute values of every deviation in B number with respect to the median (M), and normalized by dividing the median and the number of cells analyzed (N) so that the index is independent of the number of B and the sample size were performed by means of one-way ANOVA.
MI = (M-ni/fi)/M.N
where ni is the numer of B chromosome in the different types of cells that do not coincide with M, and fi is the number of cells of each particular type.
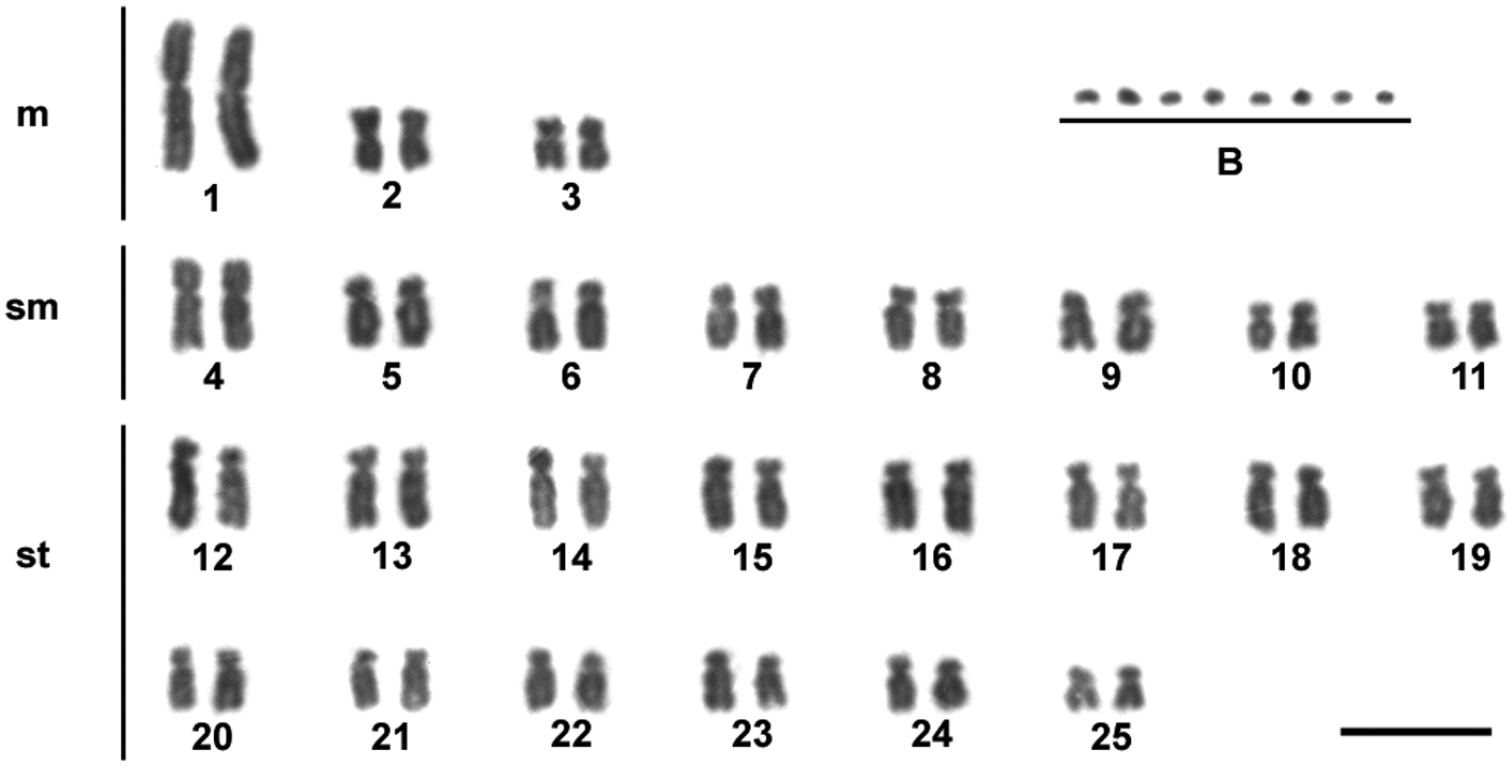
Results and discussionIn the individuals of Moenkhausia sanctaefilomenae, our results showed a diploid chromosome number of 50 chromosomes, with karyotypes composed of 6 m, 16 sm and 28 st (fundamental number FN = 100) (Fig. 1). No sex-related karyotype difference was observed. The diploid chromosome number and the karyotypes composed mainly of metacentric and submetacentric chromosomes seem to be a conserved characteristic observed for different Moenkhausia sanctaefilomenae populations (
Giemsa-stained karyotype showing 2n = 50 chromosomes of one individual of Moenkhausia sanctaefilomenae. In evidence, eight B microchromosomes. Bars = 10 µm.
Extra chromosomes were observed in the genomes of all individuals of Moenkhausia sanctaefilomenae, which were characterized as B microchromosomes (Fig. 1). We detected inter- and intra-individual variation in relation to the number of B chromosomes in the cells, with specimens bearing up to eight microchromosomes. Metaphase counts for 13 individuals showing the variation in supernumerary chromosome numbers are presented in Table 1. The modal numbers were of 2 and 3 microchromosomes. Such variation is in accordance with the pioneer study of
Metaphase counts for 13 specimens of Moenkhausia sanctaefilomenae demonstrating the variation in B microchromosome numbers.
| Specimen identification | Number of B microchromosomes per cell | Number of cells counted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 849 | 6 | 12 | 22 | - | - | - | - | - | - | 40 |
| 852 | 2 | 32 | 36 | 10 | - | - | - | - | - | 80 |
| 853 | 9 | 70 | - | - | - | - | - | - | - | 79 |
| 857 | 3 | 3 | 6 | 10 | 9 | 9 | 2 | - | - | 42 |
| 887 | - | 3 | 12 | 22 | 4 | 2 | 7 | 13 | 2 | 65 |
| 888 | - | 6 | 6 | 41 | 5 | 10 | - | - | - | 68 |
| 889 | - | 3 | 12 | 63 | 11 | 3 | - | - | - | 92 |
| 1233 | - | 4 | 10 | 24 | 26 | 29 | 13 | 4 | - | 110 |
| 1235 | 1 | 6 | 31 | 79 | 15 | 8 | - | - | - | 140 |
| 1240 | 8 | 31 | 33 | 26 | 3 | - | - | - | - | 101 |
| 1241 | 9 | 137 | 175 | 4 | - | - | - | - | - | 325 |
| 1242 | 24 | 85 | 27 | - | - | - | - | - | - | 136 |
| 1246 | 5 | 25 | 4 | 5 | 4 | 1 | - | - | - | 44 |
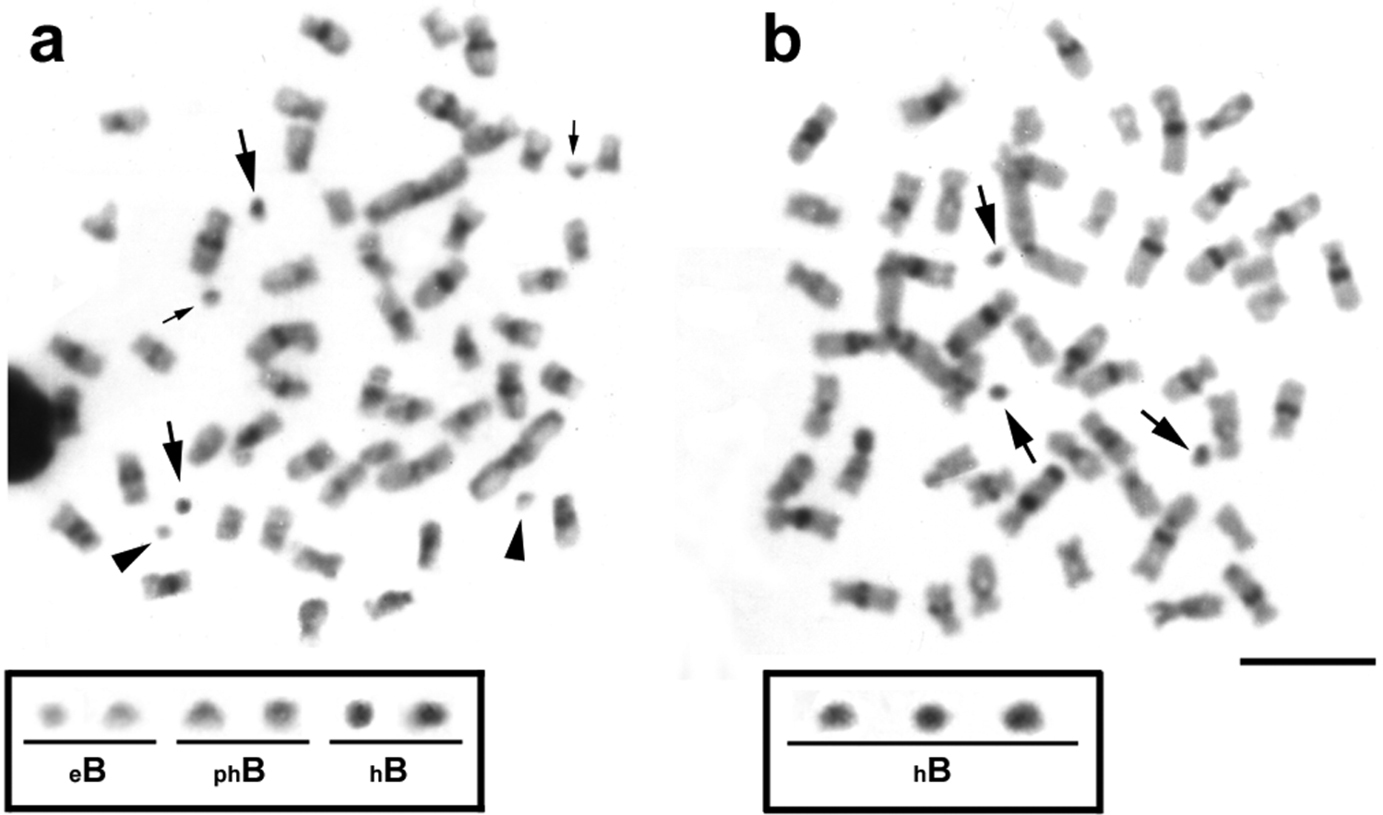
Analysis of the constitutive heterochromatin patterns by C-banding showed heterochromatic blocks in the centromeric and pericentromeric regions in the majority of the chromosomes (Fig. 2a, b). Such general heterochromatin pattern was also observed in previous analyses for other Moenkhausia sanctaefilomenae populations (
Metaphases from specimens of Moenkhausia sanctaefilomenae after C-banding technique. In (a), metaphase shows euchromatic (eB), partially heterochromatic (phB) and totally heterochromatic (hB) microchromosomes. In (b), metaphase demonstrates only heterochromatic B chromosomes. The boxes show enlarged B chromosomes. Major and minor arrows indicate totally and partially heterochromatic B microchromosomes, respectively. Arrowheads exhibit euchromatic B microchromosomes. Bars = 10 µm.
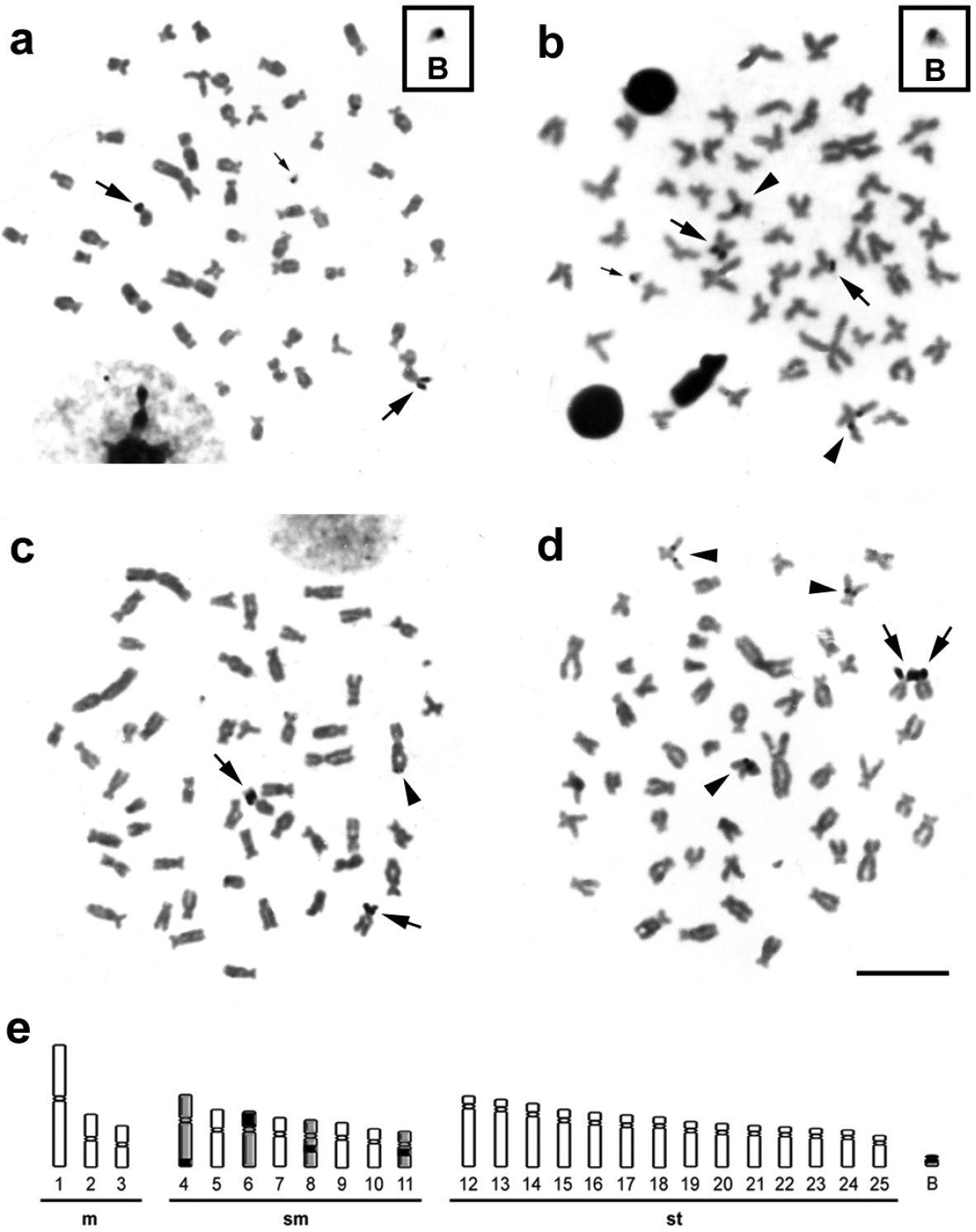
The Ag-impregnation revealed intra- (Fig. 3a, b) and inter-individual (Fig. 3c, d) variability for the NOR phenotypes in metaphases of Moenkhausia sanctaefilomenae, ranging from two to five Ag-positive sites, distributed in the interstitial and terminal regions of distinct chromosomes (Fig. 3e). However, the chromosomes 6 always presented active Ag-NORs, and consequently, were considered the major NOR-bearing chromosomes. The minor NORs showed a very variable pattern of activity. Such NOR features were previously reported by
Metaphases from specimens of Moenkhausia sanctaefilomenae submitted to the silver coloration. In (a) and (b), metaphases of one individual show intra-individual variability of active NORs. The boxes exhibit enlarged B chromosomes with nucleolar activity. In (c) and (d), metaphases of different samples demonstrate inter-individual variability for the NORs. In (e), schematic representation shows the NOR-bearing chromosomes (4, 6, 8, 11 and B). Major arrows indicate major NOR-bearing chromosomes (chromosomes 6). Minor arrows show nucleolar activity in the B microchromosomes (a) and (b). Arrowheads exhibit minor NORs demonstrating a variable pattern of activity in different chromosomes. Bars = 10 µm.
Indeed, NOR expression was detected in a B chromosome of one individual, which carried only this microchromosome (Fig. 3a, b). We analyzed 60 cells by Ag-staining and observed that about 20% had active ribosomal DNA sites in the B chromosome of this individual. Moreover, this supernumerary chromosome showed to be euchromatic by C-banding. The nucleolar region is a dynamic cell compartment involved in the control of numerous cellular functions that can be visualized after Ag coloration, when the genes present activities in the interphase that anticipates the mitosis (
The fact that the NORs located in the chromosomes 6 were always active can suggest that a process of nucleolar dominance can influence the rRNA gene transcription in order to provide the proper amount of rRNA for ribosome assembly. Nucleolar dominance is an epigenetic phenomenon common in interspecific hybrids, in which ribosomal RNA genes set inherited from one parental are rather transcribed in relation to the other (
The chromosome context appears to be important for NOR activity, as deduced from changes in the on/off activity status following chromosome rearrangements moving NORs to new locations (
In relation to the mitotic instability and maintenance of B chromosomes in Moenkhausia sanctaefilomenae, we compared the results reported by
In fish, the possibility of neutralization through mitotic stabilization of B-chromosomes was also observed in Prochilodus lineatus, in the population from the Mogi-Guaçu River (Brazil) (
In Neotropical fish, most of the studies about B chromosomes are still descriptive, because many species have not yet been cytogenetically analyzed. Thus, studies focusing B chromosomes in Neotropical fish are extremely necessary to better understand this intriguing class of chromosomes, as has been done for some species, such as Prochilodus lineatus and Astyanax species (
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).