(C) 2013 Orland J. Blanchard. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Kosteletzkya C. Presl, 1835 (Malvaceae, Malvoideae, Hibisceae) includes 17 species, all but two of which are about evenly distributed between Africa and the northern Neotropics. Fifteen of the species were brought into cultivation and used in a hybridization program in an attempt to shed light on evolutionary and phytogeographic relationships in the genus. Chromosome pairing (x = 19) at meiosis was examined in 51 of the 56 interspecific hybrids that were produced, and the seven New World species, all diploids, were found to exhibit nearly complete pairing among themselves, indicating that they share a genome. By contrast the three African diploids showed low levels of chromosome pairing in crosses among themselves, leading to the recognition here of three distinct genomes, newly designated A, B and G. The African B-genome diploid, Kosteletzkya buettneri Gürke, 1889, was found to share its genome with the New World species. Four other African species are known to be tetraploids and a fifth, a hexaploid. The results of chromosome pairing in hybrids among all of the African species at all ploidy levels, plus the discovery of a spontaneously tetraploidized experimental intergenomic African diploid hybrid, suggest that three of the four tetraploids and the single hexaploid might all be allopolyploids built on the three known extant genomes. The fourth tetraploid paired poorly or moderately with these three genomes. Results are consistent with the hypothesis that Kosteletzkya arose in Africa, radiated at the diploid level, underwent natural interspecific hybridization, produced two tiers of allopolyploids, and at some more recent time dispersed a B-genome diploid to the New World where it underwent another radiation at the diploid level. Structural features of the fruits suggest adaptations for passive distribution by animals, potentially over long distances.
Kosteletzkya, Hibisceae, Malvoideae, Malvaceae, hybridization, chromosomes, phytogeography, phylogeny
Kosteletzkya C. Presl, 1835 (Malvaceae, Malvoideae, Hibisceae) comprises 17 species that, with two exceptions, are about evenly divided between Africa (eight species) and the northern New-World tropics (seven species;
The species of Kosteletzkya are mostly herbaceous perennials that bear small to medium-sized Hibiscus-like flowers (Figs 1, 6A–P) that usually last for a single day. Indigenous uses have been reported for several of the species (
Simple gelatin-capsule device for preventing self-pollination in experimentally manipulated flowers of Kosteletzkya. a Perforated capsule-half spread with forceps in preparation for placement around the base of the style-branches of a flower of Kosteletzkya begoniifolia (76-1) b Capsule-half closed and in place between the base of the style-branches and the pollen mass. Note the recurved styles pressing their stigmas against the inside of the capsule-half.
The base chromosome number in Kosteletzkya is 19, and counts have been reported for 15 of the 17 species (
Chromosome numbers in Kosteletzkya (x = 19). Data from
| New World Species | Chromosome number (n) | African Species | Chromosome number (n) |
|---|---|---|---|
| Kosteletzkya blanchardii Fryxell, 1977 | 19 | Kosteletzkya adoensis (A. Richard, 1847) Masters, 1868 | 19 |
| Kosteletzkya depressa (Linnaeus, 1753) O. J. Blanchard, Fryxell et D. M. Bates, 1978 | 19 | Kosteletzkya buettneri Gürke, 1889 | 19 |
| Kosteletzkya hispidula (Sprengel, 1815) Garcke, 1881 | 19 | Kosteletzkya grantii (Masters, 1868) Garcke, 1880 | 19 |
| Kosteletzkya pentacarpos (Linnaeus, 1753) Ledebour, 1841 | 19 | Kosteletzkya begoniifolia (Ulbrich, 1917) Ulbrich, 1924 | 38 |
| Kosteletzkya ramosa Fryxell, 1977 | 19 | Kosteletzkya borkouana Quézel, 1957 | 38 |
| Kosteletzkya reclinata Fryxell, 1977 | 19 | Kosteletzkya rotundalata O. J. Blanchard, 2013 | 38 |
| Kosteletzkya tubiflora (de Candolle, 1824) O. J. Blanchard & McVaugh, 1978 | 19 | Kosteletzkya semota O. J. Blanchard, 2008 | 37–38 |
| Kosteletzkya racemosa Hauman, 1961 | 57 |
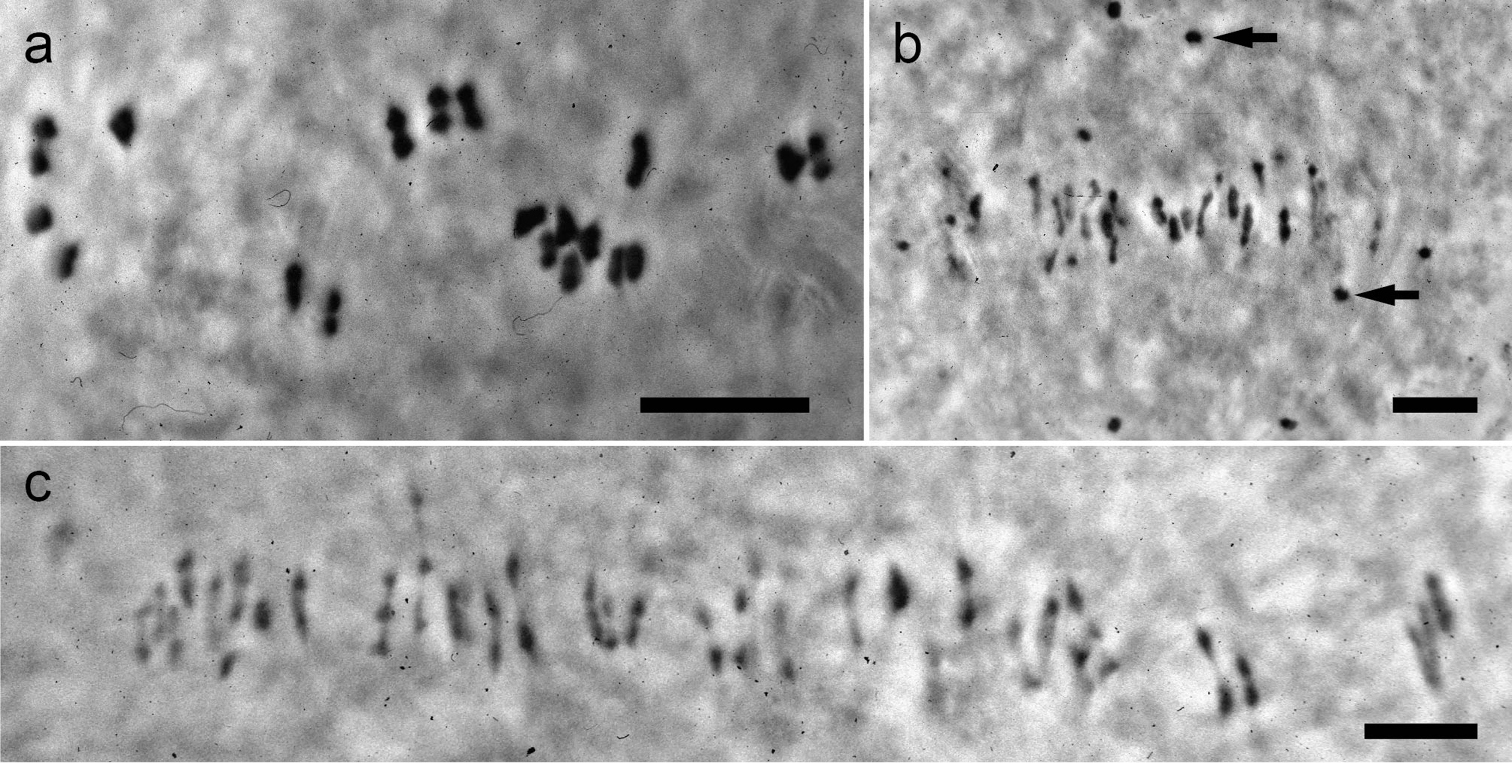
Phase-contrast photographs of meiotic metaphase I figures in two Kosteletzkya species and a tetraploid-tetraploid interspecific hybrid. a Kosteletzkya adoensis (76-36), 19IIb Kosteletzkya borkouana × Kosteletzkya begoniifolia (77-142), 22II + 32Ic Kosteletzkya borkouana (76-40), 38II. Arrows in b. indicate two of the 22 univalents. Scale bar = 10 μm.
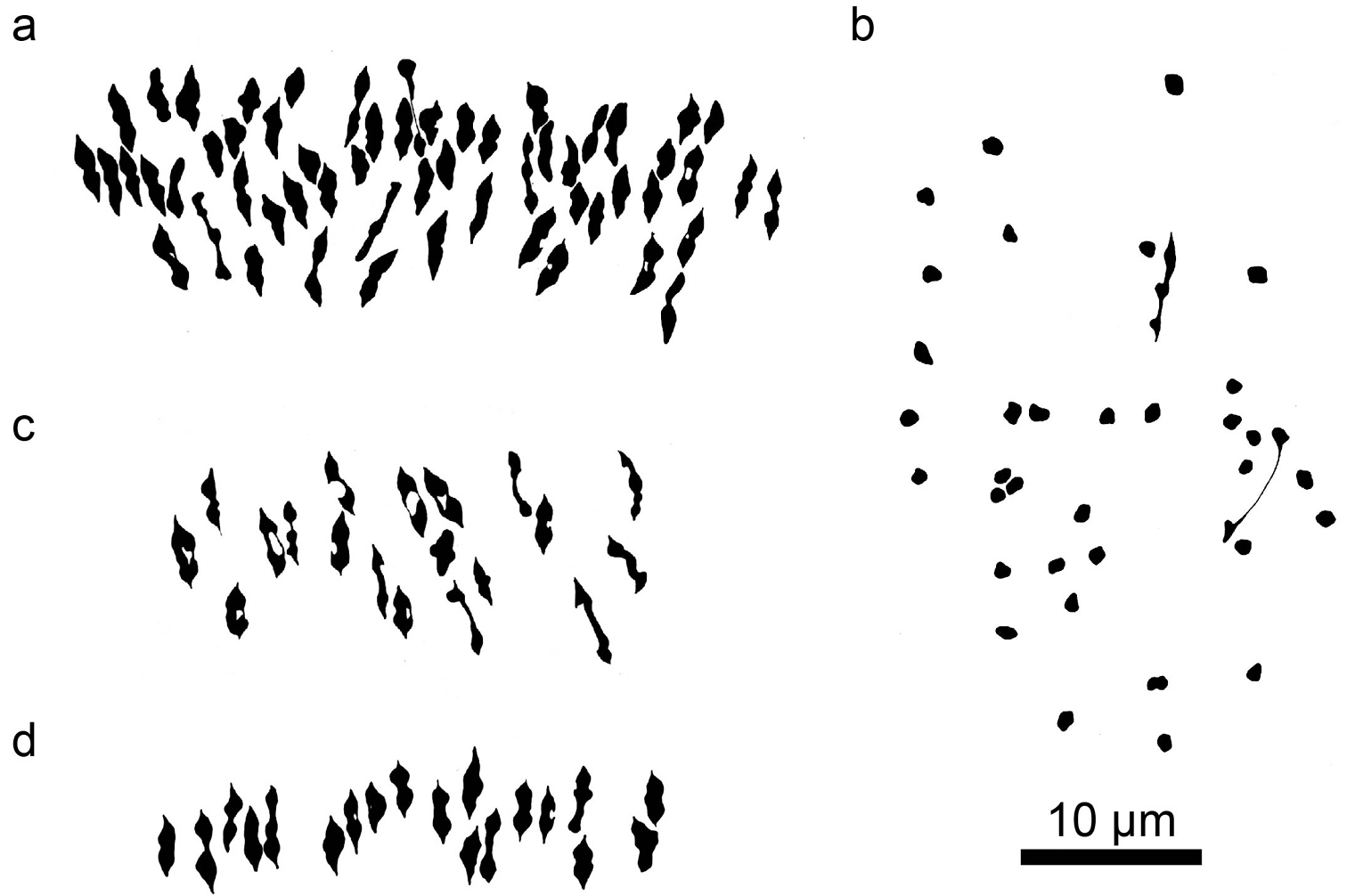
Camera lucida drawings of meiotic metaphase I figures in a species of Kosteletzkya and three diploid interspecific hybrids. a Kosteletzkya racemosa (82-88), 57IIb Kosteletzkya depressa × Kosteletzkya adoensis (77-136), 2II+34Ic Kosteletzkya depressa × Kosteletzkya tubiflora (75-149), 19IId Kosteletzkya buettneri × Kosteletzkya hispidula (Sprengel, 1815) Garcke, 1881 (75-180), 19II.
The bi-centric geographical distribution of Kosteletzkya raises a question of where the group originated. Because the more complex, polyploid-rich species assemblage in Africa suggests a longer evolutionary history than its uniformly diploid New-World counterparts, I have speculated that Africa was the birthplace of the genus (
Elsewhere in the Malvoideae, interspecific hybridization trials and the study of chromosome behavior in the resulting hybrids have been useful in clarifying species affinities, phytogeography and genomic differentiation. The two best-documented examples are the cotton genus Gossypium Linnaeus, 1753 and Hibiscus sect. Furcaria de Candolle, 1824.Each includes both diploid and polyploid species and each is distributed on both sides of the Atlantic, as well as in Australia. Gossypium comprises about 50 species (Fryxell in
Over a period of several years I have accumulated a living greenhouse collection of 15 Kosteletzkya species, and during that time I have incorporated them into a hybridization program that has attempted to shed light on the phytogeography and evolutionary history of the genus. The results are presented here. This information is expected in turn to illuminate molecular-level investigations of Kosteletzkya currently being pursued by the author and colleagues at the University of Florida.
Table 2 shows the greenhouse numbers, provenances and collectors of the 31 living accessions (i.e. cultivated progeny from a single seed source) of the 15 species used in this study, as well as six additional accessions from which the flowers in Fig. 6 were photographed.
Sources of 38 greenhouse-grown accessions of Kosteletzkya. Thirty-two accessions, representing 15 species plus an artificial tetraploid, were used in the hybridization work. Six others were sources of some of the flowers photographed for Fig. 6. Species names are followed by one or more bolded greenhouse numbers in which the year of cultivation is indicated by the two digits preceding the hyphen.
| Kosteletzkya adoensis, 74-22, ANGOLA: Huambo Distr., Instituto de Investigação Agronómica de Angola s.n.; 75-191, SIERRA LEONE: Loma Mountains, on plateau at camp 2, Morton SL 418; 76-36, ETHIOPIA: Caffa a Bonga, Saccardo 40; 10-14, ETHIOPIA: Gonder: Libo Awraja, ca. 15 km N of Addis Zemen, Tadesse and Kagnew 1973;80-107, MALAWI: N. Prov.: Nkhata Bay Dist.: 5 mi E of Mzuzu, Pawek 11872;10-31, MALAWI:Nkhata Bay Dist.: Vipya Plateau, 23 mi SW of Mzuzu, Pawek 11275; |
| Kosteletzkya “art, ” 80-103, 82-90, an artificial tetraploid, i.e. a spontaneously tetraploidized plant derived from an artificial hybrid between the two greenhouse plants Kosteletzkya adoensis 75-191 and Kosteletzkya grantii 76-2; |
| Kosteletzkya begoniifolia, 76-1, 10-64, TANZANIA: Lerai Forest, Ngorongoro Crater, Bonnefille and Riollet 73/26;79-21, ETHIOPIA: ca. 40 km W of Ambo, de Wilde and de Wilde-Duyfjes 10421; 79-53, KENYA: Seboti, SE Elgon, Tweedie 3242; |
| Kosteletzkya blanchardii, 74-10, 76-21, 88-10, 90-19, 10-26, MEXICO: Michoacán, 13 mi N of Tuzantla, Fryxell, Bates and Blanchard 1650; |
| Kosteletzkya borkouana, 76-40, 10-21, UGANDA: “Rhino Camp, ” Bahr el Jebel, Lado Enclave, Mearns 2803;79-44, CHAD: Borkou, Tigui, Quézel s.n.; 79-31, 90-36, CONGO-KINSHASA: Plaine de la Ruzizi, Lac Tsimuka, Germain 5682; |
| Kosteletzkya buettneri, 74-11, 76-22, 88-6, ZAMBIA: “C Province, ” Kafue Pontoon, Robinson 6706; 90-27, ZAMBIA: Chingola, Handlos s.n.; 90-25, 10-59, TANZANIA: Buha Dist., Malagarasi Ferry, 40 mi. from Kibondo on Kasulu road, Verdcourt 3444;79-28, 88-18, CONGO-KINSHASA: Kipopo, près d’Elisabethville (Katanga), Symoens 9242; 90-8, MALAWI: Bua River below Mude River confluence, Robson 1542; |
| Kosteletzkya depressa, 74-9, 75-136, 88-9, 90-17, MEXICO: Nayarit: 30 mi. S of Compostela, Fryxell, Bates and Blanchard 1563;10-18, MEXICO:Sinaloa: between Rosario and Esquinapa, Gentry, Barclay and Arguelles 19464; |
| Kosteletzkya grantii, 76-2, CONGO-KINSHASA: Dungu, Gérard 758; 79-41, 89-71, KENYA: Between Sio [“Soi”] River and Busia, Evans and Erens 1655;10-104, NIGERIA: Zaria: Jemaa, Sanga River Forest Reserve, Keay 37217; |
| Kosteletzkya hispidula, 74-6, 88-23, 90-39, MEXICO: Sinaloa: S of Mazatlán, Fryxell and Bates s.n.; 10-44, MEXICO: Sonora: N of El Sahuaral, Felger and Reichenbacher 85-1581; |
| Kosteletzkya pentacarpos, 74-19, USA: Florida: Seminole Co., Lake Monroe N of Sanford, Blanchard and Blanchard 306; 88-8, USA: Virginia: Chesterfield Co., N of Bermuda Hundred, Harvill 17659; 74-15, USA: Florida: Sarasota Co., Laurel, Blanchard and Blanchard 302; 10-81, IRAN: Astara [“Astava”], Wright 62; 80-142, an intraspecific hybrid between the following two greenhouse plants: 79-16, USA: Louisiana: Cameron Parish, Hackberry, Blanchard and Blanchard 423, and 79-38, IRAN: Astara [“Astava”], Wright 62; |
| Kosteletzkya racemosa, 79-24, 82-88, 90-5, 10-45, CONGO-KINSHASA: Gandajika, Liben 3266; |
| Kosteletzkya ramosa, 88-1, 10-23, MEXICO: Jalisco: 1 mi E of Ayotlán, Blanchard and Blanchard 1148; |
| Kosteletzkya reclinata, 88-15, 10-5, MEXICO: Jalisco: 11.7 km W of Tototlán, Blanchard and Blanchard 1149; |
| Kosteletzkya rotundalata, 80-104, 90-20, 10-53, CONGO-KINSHASA: Nizi, Liben 444; |
| Kosteletzkya semota, 90-2, 10-100, NIGERIA: Ogun, Omi R., Ogun Makin, Daramola s.n.; |
| Kosteletzkya tubiflora, 74-24, 90-11, 10-22, MEXICO: Jalisco: NE of Guadalajara, Barranca de los Oblatos, Fryxell, Bates and Blanchard 1590; 76-23, 78-14, MEXICO: Jalisco: K22 W of Guadalajara, Fryxell and Bates 2137. |
Plants of Kosteletzkya grow readily under glass. Uniform germination was obtained by chipping away a bit of the seed coat at the radical end of the seed. When seeds were started in the spring, these mostly short-day plants came into flower in the following fall and winter.
In most species of Kosteletzkya, a flower persists for only a single day; by early afternoon it has already begun to wither. Cross-pollinations were therefore performed by hand in the morning, shortly after the flowers had fully opened. As has been reported for Kosteletzkya pentacarpos (
A solution to this problem was found in the use of halves of gelatin capsules to separate the male and female parts of the flowers (Fig. 1a). In this technique a hole is punctured through the apex of a capsule-half using a dissecting needle that has been heated in a flame. A razor blade is then used to cut up one side of the capsule-half wall and over the top, passing through the previously cut hole. It is then possible to 1) insert the closed tines of a straight forceps into the open end of the capsule-half, 2) allow the tines to spread the razor-cut slit, 3) slip the whole unit over the base of the style branches distal to the anther mass, and then 4) allow the capsule-half to close, effectively isolating the stigmas and pollen mass from one another (Fig. 1b). In the present study each such unit was attached to a flower immediately after a cross-pollination, and the procedure proved to be highly successful in preventing self-pollination. The only drawback was that extra care had to be taken when watering the plants in order to avoid deforming or dissolving the capsule-halves.
The gelatin-capsule device could not be used with one of the species. Kosteletzkya borkouana Quézel, 1957 is effectively an obligate selfer because the stigmas have usually already recurved into the pollen mass by the time the corolla opens in the morning. To make matters more difficult, this species has the smallest flowers of any Kosteletzkya. Nevertheless it was necessary to visit the plants at 0300-0400h to carefully cut away the unopened corolla and remove the mercifully few pre-dehiscence anthers. And because the time was still hours away from the anthesis of any of the other species, actual manual cross-pollination of the emasculated flower had to await a later visit.
While most of the species that were studied were short-day, fall-and-winter-flowering plants, three of them flowered in the late summer (Kosteletzkya pentacarpos) or early fall (Kosteletzkya ramosa Fryxell, 1977 and Kosteletzkya reclinata Fryxell, 1977). To make these three available to a greater variety of other potential crossing partners, beginning in early summer plants of several of the other species were put on carts, moved daily at 1700h into an adjoining darkened room, and retrieved the next morning. Within three or four weeks, flower-bud initiation was evident. Early flowering was thereby induced so as to coincide with the flowering of the three late-summer-early-fall species.
In general, crosses worked in both directions. It appeared to make no difference in the success of an attempted cross whether a participant in the cross was the ovule-parent or pollen-parent, so no tabulated distinction is made here concerning the direction of the crosses reported. As a matter of insurance, however, the actual practice was that whenever two plants were crossed in which the size of the flowers, or more especially the style lengths, were considerably different, the smaller of the pair was used as the pollen recipient, on the theory that pollen adapted to traversing a short style might be challenged by a longer style (
Voucher specimens of most of the plants used as parents of crosses in this study, as well as specimens of the hybrids themselves, are deposited at the University of Florida Herbarium (FLAS), Florida Museum of Natural History, Gainesville, Florida, USA. In a few cases the vouchers for the parents are either the original wild-collected specimens that were the seed sources for the greenhouse plants, or they are specimens from the same seed source but grown in other years.
Pollen was stained with Cotton Blue in lactophenol. Each pollen slide was made from a single flower. Normal-sized, fully and deeply blue-stained grains were treated as “stained” and are expressed here as a percent of the total number of grains on a slide. At least three and usually five or more slides were counted for each hybrid combination. The majority of the species that participated as parents in the stainability evaluations were themselves counted and their stainabilities were found to range from 97 to 100 percent. Later in the hybridization program space was at a premium and the few replicates of hybrid plants that could be grown were used almost solely as a source of young flower buds for meiotic samples, so for some of the later-produced hybrids no fruit or pollen data were obtained. By that time, however, the general patterns of fruit-set and pollen stainability were already evident.
Recurvature of the styles is a problem for controlled pollinations, but it is a boon for fruit-set purposes because, with one exception, it was theoretically possible to let the greenhouse hybrids pollinate themselves and use those results rather than resorting to manual self-pollination. In actual practice, however, the plants were usually hand-pollinated anyway, as a part of the routine of nearly daily visits to the greenhouse.
The exception is hybrid progeny in which Kosteletzkya tubiflora (de Candolle, 1824) O. J. Blanchard & McVaugh, 1978 is one parent. This species bears distinctive yellow-and-red tubular flowers with an exserted staminal column and style (Figure 6G), and they are almost certainly bird-pollinated in the plant’s native setting. The related Kosteletzkya thurberi A. Gray, 1887 with structurally similar flowers, was reported to be visited by the Bumblebee Hummingbird (Atthis heloisa [Lesson and DeLattre, 1839]; reported as “Selasphorus heloisa”) in northern Mexico (
Fruits will set in Kosteletzkya when as few as one of the five ovules has been fertilized, and no distinction is made here as to the number of seeds in a set fruit. When an unfertilized spent flower falls, part of the pedicel remains attached to the plant, readily marking the former presence of a flower. Percent fruit-set is simply the proportion of fruit-bearing pedicels out of a total number of post-flowering pedicels.
All chromosome counts and observations of chromosome pairing were made from pollen mother cells (PMCs) at meiotic metaphase I. Details of methods of collection, fixing, staining, and preservation of meiotic material can be found in
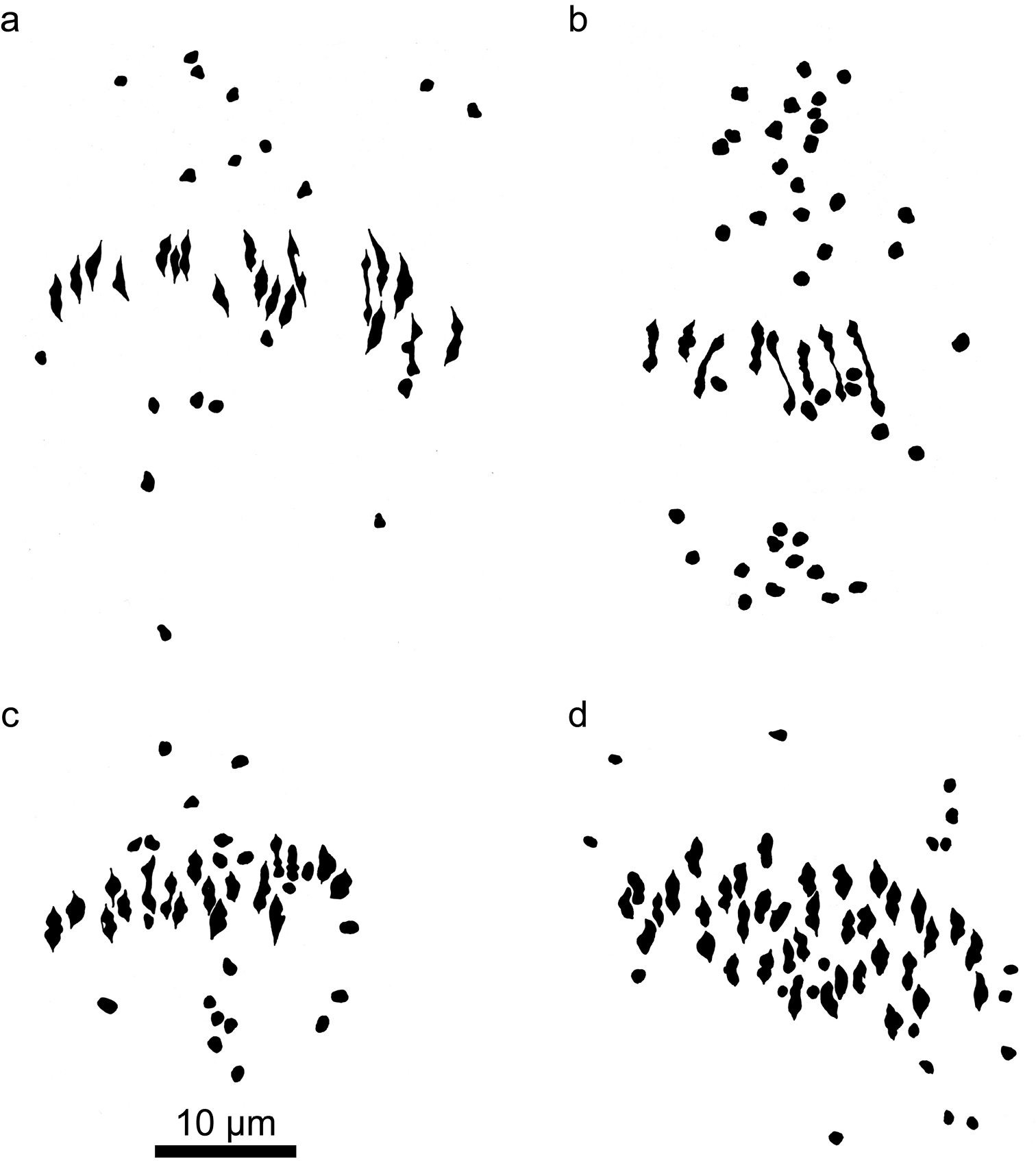
Camera lucida drawings of meiotic metaphase I figures in Kosteletzkya interspecific hybrids in which at least one parent is a polyploid. a Kosteletzkya buettneri × Kosteletzkya borkouana (77-160), 19II+19Ib Kosteletzkya grantii × Kosteletzkya borkouana (77-166), 8II+41Ic Kosteletzkya grantii × Kosteletzkya begoniifolia (81-70), 18II+21Id Kosteletzkya begoniifolia × Kosteletzkya racemosa (80-119), 38II+19I.
Camera lucida drawings of meiotic metaphase I figures in Kosteletzkya interspecific hybrids and an artificial tetraploid. a Kosteletzkya adoensis × Kosteletzkya grantii (77-153), 2II+34Ib artificial tetraploid (82-90), 38IIc Kosteletzkya artificial tetraploid × Kosteletzkya begoniifolia (81-79), 37II+2I.
A total of 56 interspecific hybrid combinations were obtained during the course of the study (Table 3). Mean values and numbers of observations are presented for percent pollen stainability, percent fruit-set and number of chromosome bivalent-equivalents in these hybrids.
Experimental crosses among species of Kosteletzkya, showing meanvalues and numbers of observations (N) for pollen stainability, fruit-set and chromosome pairing in the hybrids. Hybrids are divided into New-World, African, and trans-Atlantic crosses, plus crosses with an artificial tetraploid. Species names are abbreviated using the first three letters of the specific epithet of species listed in Table 1. Names of polyploid taxa are bolded. The maximum potential number of chromosome pairs for any particular hybrid combination is also shown, in bold, in the right-most column of the table. Note that in a few cases a particular interspecific hybrid combination was made using more than one specific pair of parent plants (see, for example, bue × sem).
| cross | parental greenhouse numbers | hybrid greenhouse number | % pollen stainability | % fruit set | bivalent equivalents | maximum potential pairing | |||
|---|---|---|---|---|---|---|---|---|---|
| mean | N | mean | N | mean | N | ||||
| NEW WORLD | |||||||||
| bla × dep | 74-9 × 74-10 | 75-144 | 42 | 7 | 20 | 584 | 19 | 16 | 19 |
| bla × his | 74-6 × 74-10 | 75-135 | 86 | 5 | 27 | 724 | 19 | 13 | 19 |
| bla × pen | 74-19 × 74-10 | 75-159 | 15 | 6 | 2 | 127 | 18.9 | 10 | 19 |
| bla × ram | 88-10 × 88-1 | 89-5 | 90 | 3 | 7 | 71 | 19 | 6 | 19 |
| bla × tub | 74-24 × 4-10 | 75-161 | 56 | 6 | 25 | 4 | 18.7 | 6 | 19 |
| dep × pen | 74-19 × 4-9 | 75-150 | 25 | 5 | 14 | 224 | 18.5 | 13 | 19 |
| dep × his | 74-9 × 74-6 | 75-178 | 39 | 5 | 41 | 758 | 18.9 | 11 | 19 |
| dep × ram | 88-9 × 88-1 | 89-11 | 39 | 3 | 26 | 186 | - | - | |
| dep × rec | 88-9 × 88-15 | 89-12 | 51 | 3 | 49 | 390 | 19 | 3 | 19 |
| dep × tub | 74-9 × 74-24 | 75-149 | 51 | 5 | 29 | 143 | 19 | 25 | 19 |
| his × pen | 74-6 × 74-15 | 75-127, 75-157 | 79 | 5 | 11 | 210 | 18.9 | 14 | 19 |
| his × ram | 88-23 × 88-1 | 89-7 | 95 | 3 | 65 | 147 | - | - | |
| his × rec | 88-23 × 88-15 | 89-1 | 94 | 3 | 54 | 247 | 19 | 8 | 19 |
| his × tub | 74-6 × 74-24 | 75-151 | 91 | 6 | 49 | 138 | 19 | 17 | 19 |
| pen × rec | 88-8 × 88-15 | 89-6 | 78 | 3 | 38 | 343 | 18.8 | 9 | 19 |
| pen × tub | 74-19 × 74-24 | 75-168 | 73 | 6 | 31 | 88 | 18.9 | 9 | 19 |
| rec × ram | 88-15 × 88-1 | 89-3, 89-9 | 98 | 6 | 66 | 154 | 18.9 | 7 | 19 |
| AFRICA (polyploid species bolded) | |||||||||
| ado × bue | 76-36 × 76-22 | 77-145 | 0 | 5 | 0 | 113 | 3.1 | 10 | 19 |
| ado × gra | 75-191 × 76-2 | 77-153 | 1 | 5 | 0 | 37 | 2.0 | 25 | 19 |
| bue × gra | 76-2 × 76-22 | 77-158 | 0 | 5 | 0 | 112 | 9.1 | 17 | 19 |
| bue × beg | 76-22 × 76-1 | 77-100, 81-26 | 0 | 5 | 0 | 252 | 3.9 | 25 | 19 |
| 79-28 × 79-53 | 81-31 | - | - | 0 | 218 | - | - | ||
| bue × bor | 76-22 × 76-40 | 77-160 | 0 | 5 | 0 | 21 | 18.8 | 12 | 19 |
| bue × sem | 90-27 × 90-2 | 91-34 | - | - | - | - | 8.5 | 6 | 19 |
| 90-8 × 90-2 | 91-39 | - | - | - | - | 4.4 | 25 | 19 | |
| 90-25 × 90-2 | 91-10 | - | - | - | - | 6.1 | 15 | 19 | |
| gra × beg | 79-41 × 79-21 | 81-70, 81-74 | 2 | 3 | 0 | 247 | 17.9 | 15 | 19 |
| gra × bor | 76-2 × 76-40 | 77-166 | 0 | 5 | 0 | 10 | 7.0 | 3 | 19 |
| gra × sem | 90-2 × 89-71 | 91-37 | - | - | - | - | 13.1 | 9 | 19 |
| beg × bor | 76-1 × 76-40 | 77-142 | 5 | 5 | 0 | 171 | 24.6 | 14 | 38 |
| beg × rot | 79-53 × 80-104 | 81-76 | 97 | 4 | 63 | 182 | 37.0 | 2 | 38 |
| bor × sem | 90-36 × 90-2 | 91-8 | - | - | - | - | 3.5 | 15 | 37-38 |
| rot × sem | 90-2 × 90-20 | 91-4 | - | - | - | - | 11.3 | 20 | 37-38 |
| beg × rac | 79-21 × 79-24 | 80-119 | 42 | 5 | 0 | 234 | 37.1 | 9 | 38 |
| bor × rac | 79-44 × 79-24 | 80-115, 80-116 | 5 | 5 | 0 | 671 | 37.8 | 4 | 38 |
| 79-31 × 79-24 | 80-117 | - | - | 0 | 147 | - | - | ||
| rot × rac | 80-104 × 79-24 | 81-73 | 33 | 3 | 0 | 141 | - | - | |
| sem × rac | 90-2 × 90-5 | 91-3 | - | - | - | - | 6.6 | 11 | 37-38 |
| TRANS-ATLANTIC (African species listed first; polyploid species bolded) | |||||||||
| ado × dep | 74-22 × 74-9 | 75-130, 75-156, 77-136 | 7 | 8 | 1 | 143 | 1.2 | 13 | 19 |
| bue × bla | 74-11 × 74-10 | 75-145 | 26 | 10 | 1 | 155 | 19 | 10 | 19 |
| bue × dep | 74-11 × 74-9 | 75-104 | 32 | 14 | 2 | 264 | 18.9 | 13 | 19 |
| bue × his | 74-11 × 74-6 | 75-180 | 37 | 5 | 8 | 245 | 19 | 10 | 19 |
| bue × pen | 74-11 × 74-19 | 75-148 | 27 | 7 | 0 | 129 | 19 | 11 | 19 |
| bue × ram | 88-18 × 88-1 | 89-8 | 74 | 3 | 14 | 29 | 18.9 | 8 | 19 |
| bue × rec | 88-6 × 88-15 | 89-4 | 35 | 3 | 0 | 47 | - | - | |
| bue × tub | 79-28 × 78-14 | 81-91 | 61 | 3 | 4 | 75 | 19 | 5 | 19 |
| gra × bla | 76-2 × 76-21 | 77-113 | 0 | 5 | 0 | 791 | 11.4 | 10 | 19 |
| gra × dep | 76-2 × 75-136 | 77-115 | - | - | - | - | 7.7 | 13 | 19 |
| gra × pen | 79-41 × 80-142 | 81-159 | 0 | 3 | 0 | 75 | - | - | |
| beg × bla | 76-1 × 76-21 | 77-104 | 1 | 4 | 0 | 272 | 6.1 | 14 | 19 |
| bor × dep | 76-40 × 75-136 | 77-183 | 4 | 5 | 0 | 67 | 18.0 | 1 | 19 |
| bor × bla | 76-40 × 76-21 | 77-167 | - | - | - | - | 18.6 | 11 | 19 |
| bor × tub | 76-40 × 76-23 | 77-173 | 0 | 5 | 0 | 17 | 18.7 | 6 | 19 |
| sem × bla | 90-2 × 90-19 | 91-1 | - | - | - | - | 4.3 | 19 | 19 |
| sem × his | 90-2 × 90-39 | 91-35 | - | - | - | - | 7.0 | 17 | 19 |
| sem × tub | 90-2 × 90-11 | 91-36 | - | - | - | - | 10.9 | 15 | 19 |
| CROSSES WITH ARTIFICIAL TETRAPLOID (polyploid taxa bolded) | |||||||||
| art × ado | 80-103 × 80-107 | 81-89 | - | - | 19 | 4 | 19 | ||
| art × gra | 80-103 × 79-41 | 81-87 | - | - | 19 | 5 | 19 | ||
| art × beg | 80-103 × 79-53 | 81-79 | - | - | 36.9 | 13 | 38 | ||
| art × rot | 80-103 × 80-104 | 81-83 | - | - | 36.6 | 15 | 38 | ||
All seven available New World species were involved in the crossing program (an eighth species, Kosteletzkya thurberi, was unavailable), and all interspecific crosses that were attempted were successful and comprised 17 of the 21 possible pairwise combinations among the seven species. Hybrid plants generally grew as vigorously as their parents under greenhouse conditions. Pollen stainability among them ranged from 15 to 98 percent, while fruit-set ranged from 2 to 66 percent. Low or high values in one measure did not necessarily correspond with those of the other measure. For instance, the combination Kosteletzkya blanchardii Fryxell, 1977 × Kosteletzkya ramosa had 90 percent pollen stainability but only 7 percent fruit-set.
Despite wide morphological differences among the New-World species, nearly complete chromosome pairing, as indicated by the average number of bivalents, was found in each of the 15 hybrid combinations that were examined meiotically (Table 3). Average values ranged from 18.5 to 19 bivalents out of a possible 19. As an example, Fig. 3c shows a meiotic metaphase figure from the hybrid Kosteletzkya depressa (Linnaeus, 1753) O. J. Blanchard, Fryxell et D. M. Bates, 1978 × Kosteletzkya tubiflora, whose parental species are dramatically different in habitat, morphology and floral adaptations. Kosteletzkya depressa is a lowland, bee-pollinated plant with a small, white-to-pink, rotate corolla (petals 0.8–1 cm long), included staminal column, and a green calyx (Fig. 6B); Kosteletzkya tubiflora is an upland, apparently bird-pollinated plant with a large, yellow, tubular corolla (petals 2.5–3 cm long), exserted staminal column and a pink-to-red calyx (Fig. 6G).The two species also differ markedly in fruit and seed characteristics.
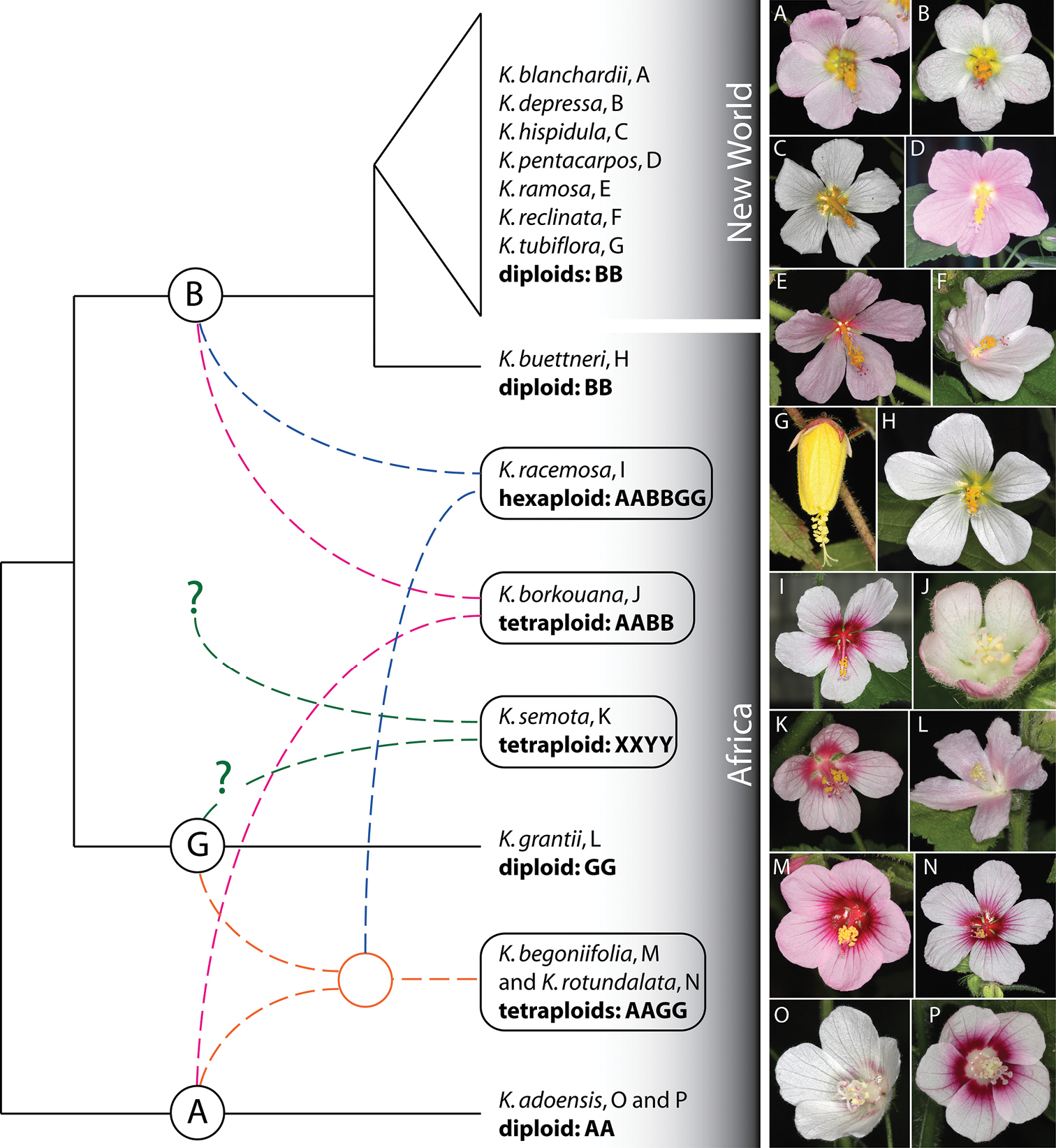
A representation of hypothesized phylogenetic and phytogeographic relationships among 15 species of Kosteletzkya based on chromosome pairing in experimental hybrids, plus photographs of the 15 species. Extant allopolyploids are shown in boxes. Distinct genomes are identified by the letters A, B, G, X and Y. Differently colored dashed lines indicate how these ancestral parents are thought to have combined to form the present-day polyploid species. One of the two alternate derivations of Kosteletzkya racemosa—that in which the B genome is carried by the diploid ancestor—is shown here. Question marks indicate uncertainties about the origins of the postulated diploid ancestors of Kosteletzkya semota. Letters after the names of taxa refer to the flower photographs to the right. The latter are not all shown to the same scale. Plants from which the flower images were made are indicated by the following greenhouse numbers (see Table 2): A 10-26 B 10-18 C 10-44 D 10-81 E 10-23 F 10-5 G 10-22 H 10-59 I 10-45 J 10-21 K 10-100 L 10-104 M 10-64 N 10-53 O 10-14 P 10-31.
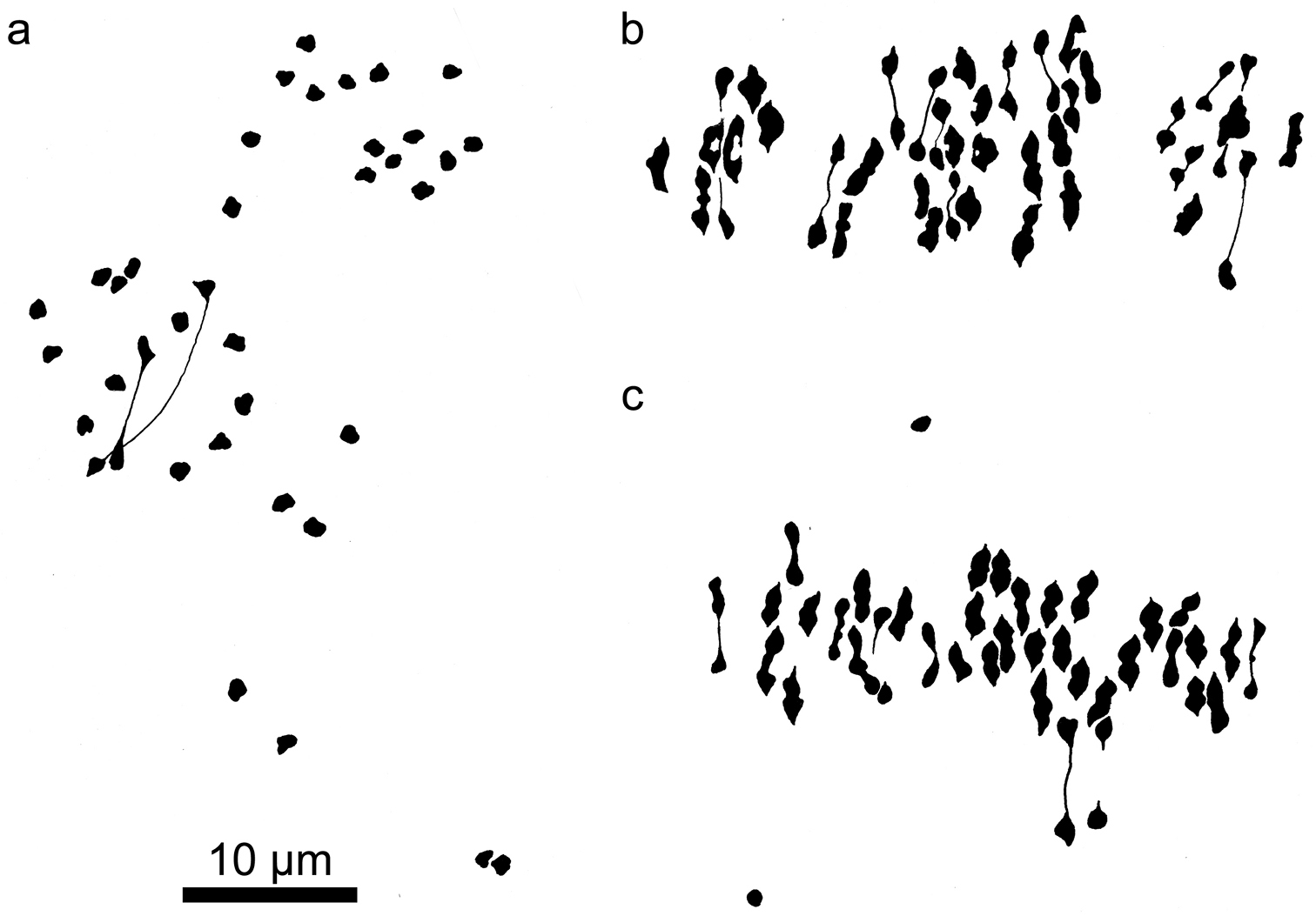
All three possible hybrids among the three known African diploid species Kosteletzkya adoensis (A. Richard, 1847) Masters, 1868, Kosteletzkya buettneri and Kosteletzkya grantii (Masters, 1868) Garcke, 1880 were obtained, although these offspring were not as robust as the New-World hybrids. Like the New-World diploid species, the three African parent species differ considerably in habit, leaf shape, inflorescence form and details of flowers (see Figs 6H, L, O and P), fruits and seeds. However in dramatic contrast to the diploid New-World hybrids, pollen stainability in the African diploid hybrids ranged from 0 to 1 percent, fruit-set was 0 percent, and average chromosome pairing ranged from 2.0 to 9.1 out of a potential 19 bivalent-equivalents. A meiotic metaphase I of the hybrid Kosteletzkya adoensis × Kosteletzkya grantii is shown in Fig. 5a.
Hybrids between African diploids and African tetraploids were generally obtained with more difficulty, particularly in the case of the diploid Kosteletzkya adoensis, in which, to cite the most extreme example, several hundred cross-pollinations with Kosteletzkya begoniifolia (Ulbrich, 1917) Ulbrich, 1924 resulted in only a single viable seed. Nevertheless, altogether six of the possible 12 diploid-tetraploid hybrids were eventually produced. As might be expected in hybrids between ploidy levels, pollen stainability was low (0-2 percent in the four interspecific combinations that were sampled for this characteristic) and their fruit-set was likewise low (0 percent in the same four combinations). Average chromosome pairing in these six hybrids varied widely. Two approached the potential maximum of 19, forming 17.9 to 18.8 pairs (Figs 4a and 4c), while the other four ranged in average from 3.9 to 13.1 bivalent-equivalents (Fig. 4b).
No diploid-hexaploid hybrids could be obtained despite numerous cross-pollinations.
The African polyploids could be crossed fairly easily among themselves, and eight of the ten possible hybrids were produced. In the five cases where pollen stainability and fruit-set were determined, stainability ranged from 5 to 97 percent, whereas fruit-set for four of the hybrids was 0 percent, while for a fifth (Kosteletzkya begoniifolia × Kosteletzkya rotundalata) it was 63 percent. Of the four tetraploid-tetraploid hybrids that were examined cytologically, one had bivalent-equivalents approaching the maximum possible 38, whereas the other three ranged from 3.5 to 24.6 bivalent-equivalents (Fig. 2b). Of the three tetraploid-hexaploid hybrids examined cytologically, two averaged over 37 pairs out of a potential maximum of 38 (Fig. 4d), while the third averaged only 6.5 bivalent-equivalents.
Eleven of the 21 possible diploid-diploid trans-Atlantic combinations were produced. At least four failed attempts involved Kosteletzkya adoensis as one potential parent. One combination that was obtained, Kosteletzkyagrantii × Kosteletzkya depressa, produced flower buds that aborted between meiosis and flowering, making pollen stainability and fruit-set data impossible to obtain. In another combination, Kosteletzkya adoensis × Kosteletzkya depressa, though the plants flowered, they were weak-stemmed and slow-growing, and produced only a feeble root system. The 10 surviving hybrids had pollen stainabilities and fruit-sets that were intermediate, on average, between those of New World diploid-diploid crosses and those of the African diploid-diploid crosses, and ranged from 0 to 74 percent pollen stainability and 0 to 14 percent fruit-set. However, depending on which of the African diploids participated, the pollen-stainability outcomes were different: in the seven African-New World crosses involving Kosteletzkya buettneri, stainability ranged from 26 to 74 percent; in the two crosses involving Kosteletzkya grantii, stainability was zero percent in both cases; and in the single cross involving Kosteletzkya adoensis, the result was seven percent pollen stainability. Outcomes of chromosome pairing observations were even more distinctly different depending on which African parent was involved. In all six combinations in which Kosteletzkya buettneri was the African parent, pairing closely approached the maximum possible 19 (see for example Fig. 3d). However when Kosteletzkya grantii or Kosteletzkya adoensis were involved, the pairing in the three hybrids that were examined cytologically ranged from 1.2 to 11.4 bivalent-equivalents (see for example Fig. 3b).
In the case of trans-Atlantic crosses between African tetraploids and New-World diploids there was again a bimodal pattern. In three of the seven hybrid combinations examined meiotically, chromosome pairing approached the maximum 19; in the other four the range was 4.3 to 10.9.
As was the case for African-African crosses, no diploid-hexaploid trans-Atlantic combinations could be obtained despite numerous crossing attempts.
In clear contrast to the nearly perfect chromosome pairing (18.5–19 bivalent-equivalents) in all of the 17 diploid New-World hybrids, the three African diploids contain chromosome sets with only low-to-modest affinity among themselves (2.0, 3.1 and 9.1 bivalent-equivalents). A consequence of this is mirrored in the negligible pollen stainability (0 to 1 percent) and zero fruit-set in hybrids among the three African species. This has prompted the designation here of three distinct genomes among the African diploids: A for the Kosteletzkya adoensis genome, B for the Kosteletzkya buettneri genome and G for the Kosteletzkya grantii genome.
Considering chromosome-pairing relationships in these genomic terms, it is apparent that only one genome is shared by all of the New World species. More interestingly, the trans-Atlantic crosses between the African diploid Kosteletzkya buettneri and six different species from the New World show a nearly perfect pairing in each, consisting on average of 18.9 to 19 bivalent-equivalents. Clearly then, the one New-World genome must be B. Indirect support for this comes from the fact that New-World species recognize only 7.7 to 11.4chromosomes in the G genome, and only 1.2 chromosomes in the A genome—a pattern similar to crosses of these same two genomes directly with Kosteletzkya buettneri itself. These results, of course, indicate a direct connection between the African and New World parts of the genus. Simply stated, the African Kosteletzkya buettneri appears to be more closely related to the New World species than it is to its two African diploid congeners.
Allopolyploidy is an important mechanism for speciation in plants (
In classic allopolyploidy, the production of an interspecific hybrid is the first step leading to a new species, yet in an examination of over 2800 herbarium specimens comprising all 17 species in the genus, no plants were found that might have been considered natural hybrids. In a way, this is not a surprise. The New-World species, though relatively easily inter-crossable, are at present largely geographically allopatric, and even where they are in geographic proximity they are kept separate elevationally (e.g. Kosteletzkya depressa and Kosteletzkya tubiflora in western Mexico) or by flowering season (e.g. Kosteletzkya depressa and Kosteletzkya pentacarpos in western Cuba and southern Florida). In contrast, while the African diploids are broadly sympatric, though perhaps often ecologically separated, experiments reported here have shown that hybrids among them are more difficult to obtain and weaker—conditions likely to pertain in the wild as well. In either hemisphere the hybrids themselves would obviously be transitory, living for a few years and then likely vanishing unrecognized and leaving few if any progeny.
Nevertheless, there appears to be clear evidence of allopolyploidy in Africa. The experimental diploid-tetraploid hybrid between Kosteletzkya grantii (2x) and Kosteletzkya begoniifolia (4x) averaged 17.9 bivalent-equivalents out of a possible 19, and likewise the diploid-tetraploid hybrid between Kosteletzkya buettneri (2x) and Kosteletzkya borkouana (4x) averaged 18.8 out of 19. The reverse crosses (Kosteletzkya buettneri × Kosteletzkya begoniifolia and Kosteletzkya grantii × Kosteletzkya borkouana) yielded averages of 3.9 and 7.0 respectively. This suggests that the G genome but not the B genome is present in the tetraploid Kosteletzkya begoniifolia, and the B genome but not the G genome is present in the tetraploid Kosteletzkya borkouana. Neither of these two diploids was combined in this study into a hybrid with the tetraploid Kosteletzkya rotundalata but this species shows nearly complete chromosome homology with Kosteletzkya begoniifolia—an average of 37.0 pairs out of a potential 38—suggesting a close relationship between the two, and indirectly indicating that the G genome is also present in Kosteletzkya rotundalata. Finally, the tetraploid Kosteletzkya semota was combined with both the B-bearing Kosteletzkya borkouana and the G-bearing Kosteletzkya rotundalata, producing on average only 3.5 and 11.3 pairs respectively, from which it can be reasonably concluded that Kosteletzkya semota contains neither B nor G genomes. This is further suggested by crosses of Kosteletzkya semota with the African diploids Kosteletzkya buettneri and Kosteletzkya grantii as well as with three New-World B-genome diploids, in which all five results ranged between 4.3 and 13.1 bivalent-equivalents.
When the tetraploids Kosteletzkya begoniifolia and Kosteletzkya borkouana were crossed with one another, the resulting hybrids averaged 24.6 bivalent-equivalents out of a possible 36, which indicates that they share a genome. That genome cannot be either G or B since it is shown above that neither is shared by the two tetraploids. This shared, unknown Kosteletzkya borkouana-Kosteletzkya begoniifolia-Kosteletzkya rotundalata genome cannot be present in Kosteletzkya semota because many fewer that a full set of 19 chromosomes were detected in Kosteletzkya semota by these other tetraploids.
Although one might invoke some undiscovered or now-extinct genome as the postulated shared genome, the most obvious suggestion is that it is the extant genome A. It was therefore particularly frustrating that hundreds of cross-pollinations between the A-bearing Kosteletzkya adoensis and the tetraploids Kosteletzkya begoniifolia and Kosteletzkya borkouana produced only a single viable seed—from the first of these two combinations—and that seed yielded a severely stunted, deformed, non-reproductive plant. This made it impossible to introduce an A genome into either of the two tetraploids to the extent that it could reach meiosis and seek out a possible genomic match. On the other hand, the putative hybrids that would have had to form in nature as a first step in the allopolyploid production of the postulated AAGG and AABB tetraploids are, respectively, the combinations AG (Kosteletzkya adoensis × Kosteletzkya grantii), and AB (Kosteletzkya adoensis × Kosteletzkya buettneri). Both of these hybrids were indeed produced experimentally. The first had 1 percent pollen stainability, 0 percent fruit-set and produced an average of 2.0 bivalent-equivalents out of a potential 19; the second had 0 percent pollen stainability, 0 percent fruit-set, and an average of 3.1 bivalent equivalents. Fig. 5a illustrates a meiotic metaphase I of the AG hybrid.
Remarkably, one day I found in the greenhouse a normal-appearing fruit on a branch of the otherwise profoundly sterile diploid hybrid AG plant. It contained three well-formed seeds, all of which subsequently germinated and produced vigorous plants which themselves yielded abundant fruit when selfed. This restoration of fertility strongly suggested that a spontaneous doubling of chromosomes had occurred in at least a small part of the hybrid plant, thereby creating identical pairs of chromosome sets and permitting full synapsis in meiotic prophase I, with the result that meiosis and gamete formation were able to proceed to normal completion. Examination of meiotic metaphase in one of these plants indeed showed 38 pairs of chromosomes (Fig. 5b). The conclusion: a combination of experimental and accidental events had resulted in a new, fully fertile tetraploid. A test of this would be a cross between this artificial tetraploid and one of its wild putative counterparts, Kosteletzkya begoniifolia or Kosteletzkya rotundalata. Both of these test hybrids were obtained, and they showed averages of 36.9 and 36.6 bivalent-equivalents respectively out of a potential 38. Fig. 5c shows a meiotic figure illustrating the combination Kosteletzkya begoniifolia × Kosteletzkya artificial tetraploid.
This settled two important matters: 1) that the artificial tetraploid corresponded genomically to these wild tetraploids, and 2) that the identity of the elusive shared genome was indeed A. The latter was also separately verified by subsequent backcrossing of the artificial tetraploid to each of its two diploid parents. These crosses with Kosteletzkya adoensis and with Kosteletzkya grantii each yielded an average pairing of 19 out of 19 potential pairs. Interestingly, Kosteletzkya adoensis, which had been so intractable in attempts at crossing it with the two wild tetraploids, crossed fairly readily with their home-made counterpart, and the offspring grew and flowered well.
In summary, these results suggest that the genomic makeups of the tetraploids Kosteletzkya begoniifolia, Kosteletzkya rotundalata and Kosteletzkya borkouana are respectively AAGG, AAGG and AABB.
Since it is generally observed that most allopolyploids arise via unreduced gametes (
Despite numerous attempts, no hybrids could be produced between the single African hexaploid Kosteletzkya racemosa and any of the diploids, either African or New-World, however the hexaploid did cross with all four tetraploids. With Kosteletzkya begoniifolia and Kosteletzkya borkouana it averaged 37.1 and 37.8 chromosome pairs respectively out of a possible 38 (Figure 4d), whereas with Kosteletzkya semota it showed 6.6 pairs out of a possible 38. (Meiotic material from the hybrid Kosteletzkya rotundalata × Kosteletzkya racemosa was not obtained.) Now that genomic makeups are known for the tetraploids Kosteletzkya borkouana and Kosteletzkya begoniifolia it can be stated with reasonable certainty that the genomic constitution of the hexaploid is AABBGG. This is because both AAGG (Kosteletzkya begoniifolia) and AABB (Kosteletzkya borkouana) tetraploids were shown separately to find nearly perfect correspondence with two sets of chromosomes in the hexaploid.
The hexaploid could theoretically have arisen in nature in one of two ways. A triploid ABG hybrid could have formed between an AABB tetraploid and a GG diploid, or between an AAGG tetraploid and a BB diploid, in both cases followed by chromosome doubling. No persuasive evidence at present strongly favors either scenario as being more likely, but the Kosteletzkya buettneri-like narrower leaves and depressed fruit, plus the Kosteletzkya begoniifolia-like dark petal bases and larger seeds that together characterize the hexaploid, hint that the combination AAGG × BB might be the better candidate. The matter is complicated by the fact that while both of the relevant diploids occur in the geographical vicinity of the two known occurrences of Kosteletzkya racemosa in southern Congo-Kinshasa and northwestern Zambia, none of the tetraploids are currently known to do so.
The preceding discussion is not meant to imply that Kosteletzkya adoensis, Kosteletzkya buettneri and Kosteletzkya grantii are the direct, immediate sources of the genomes A, B and G that are found in the polyploids, but rather that the lineages that gave rise to these three modern diploids also contributed their genomes relatively recently to the polyploids. The two interspecific hybrids that are the diploid counterparts, AB and AG, of the natural tetraploids whose genomic makeups are known, i.e. Kosteletzkya borkouana (AABB) and Kosteletzkya begoniifolia/rotundalata (AAGG), are only somewhat similar morphologically, not identical, to these tetraploids. Likewise the artificial tetraploid that is the full genomic counterpart of the wild species Kosteletzkya begoniifolia is similar to the latter, but distinguishable from it. Finally, both the triploid combination Kosteletzkya begoniifolia-Kosteletzkya buettneri (ABG), and the triploid combination Kosteletzkya borkouana-Kosteletzkya grantii (also ABG), look similar, but not identical, to the hexaploid Kosteletzkya racemosa (AABBGG).
Kosteletzkya semota, the third genomically distinctive tetraploid (recall that Kosteletzkya rotundalata and Kosteletzkya begoniifolia are genomically alike), appears to show little affinity with any of the three known diploid genomes. In effect the hexaploid Kosteletzkya racemosa offered all three known genomes to Kosteletzkya semota in a cross, but the 37-38 chromosomes of the latter could only recognize, on average, 6.6 Kosteletzkya racemosa chromosomes—the equivalent of about one-third of a genome. There were similar outcomes when semota participated in crosses with other tetraploids discussed above, yielding bivalent-equivalents ranging from 3.5 to 11.3, indicating at best a low-to-modest level of chromosomal homology with any of the other known genomes. Interestingly however, Kosteletzkya semota was shown to share over two-thirds of a genome (13.1 pairs) in a cross directly with the diploid Kosteletzkya grantii. These varied results leave uncertainties about the evolutionary position Kosteletzkya semota, so it seems best to give it a provisional designation of XXYY, which recognizes the species as distinctive and assumes an allopolyploid origin. At least one of its diploid progenitors, and its constituent genome, remains undiscovered or, more likely, extinct.
The decision to convert trivalents and quadrivalents to bivalent-equivalents (see Materials and methods) was intended to make Table 3 easier to read and interpret, but it is worth considering whether this conversion might have led to bias. In polyploid hybrids, multivalent associations sometimes indicate pairing within genomes (autosyndesis), which in turn suggests autopolyploidy—an interpretation that conflicts with the hypothesis of allopolyploidy that I have posited here. In the present case however, such an explanation is highly unlikely. Of the 618 meiotic cells examined, only nine had a single trivalent and only 18 others had a single quadrivalent. Moreover, 24 of these 27 multivalents occurred in diploid-diploid hybrids, and therefore could not be attributed, by definition, to autosyndesis. The three exceptions, involving one trivalent and two quadrivalents, were all found in hybrids in which the artificial tetraploid was one parent, and since I have shown here that this plant was derived from an interspecific diploid-diploid hybrid, its multivalents cannot be interpreted as indicating autopolyploidy.
Figure 6 depicts a reconstruction of the postulated genomic-phytogeographic history of the genus Kosteletzkya based on the cytogenetic evidence presented here. It assumes that the degree of chromosome pairing in the experimental hybrids can be used as a rough relative measure of the degree of evolutionary divergence of the parents of a cross. In support of this assumption, I note that in the well-studied malvaceous genus Gossypium, the African genomes A, B and E show pairing relationships among themselves (data from
The reconstruction shown here indicates the reticulate nature of the evolution of Kosteletzkya in Africa, and also emphasizes that all of the early events in the history of the genus took place on the African continent. The lineage giving rise eventually to the B and G genomes is shown as separating from the A-genome lineage early in the evolution of the genus. This B-G branch itself branched in a more recent step, leading eventually to the extant African diploids Kosteletzkya buettneri and Kosteletzkya grantii respectively, while the A genome eventually gave rise to the extant diploid Kosteletzkya adoensis. The rest of the African diversification occurred at the polyploid level, and initially involved two separate interspecific hybridizations, each of which involved an A-genome plant—one in combination with a B-genome plant and the other with a G-genome plant. Following a doubling of the chromosome complements in these two hybrids, and subsequent evolution at the tetraploid level, the two resulted in three extant species: Kosteletzkya borkouana with genomic makeup AABB, and Kosteletzkya begoniifolia and Kosteletzkya rotundalata, each with AAGG. A cross between one of these tetraploids and a diploid bearing the third genome, followed by doubling, produced the hexaploid Kosteletzkya racemosa having a genomic makeup of AABBGG. Of the two possible hybrid combinations that might have led to this hexaploid, I have illustrated the one in which the tetraploid partner is from the Kosteletzkya begoniifolia-Kosteletzkya rotundalata (AAGG) lineage, since this alternative seems better supported by morphological evidence. The two postulated early-diverging genomes that led to the formation of the tetraploid Kosteletzkya semota have been designated here as XXYY, but in the depiction in Fig. 6, one of its genomes is tentatively shown as having its origin in the Kosteletzkya grantii lineage, since the cross Kosteletzkya semota × Kosteletzkya grantii yielded 13.1 chromosome pairs, the equivalent of more than 2/3 of a genome.
The atypically high level of fruit-set—63 percent—in the African tetraploid-tetraploid cross Kosteletzkya begoniifolia × Kosteletzkya rotundalata contrasts dramatically with the zero percent seen in all twelve of the other African hybrids for which there are fruit-set data (Table 3). This and the pairing evidence imply that the two parents diverged relatively recently from a common tetraploid ancestor, and this, too, is suggested in Fig. 6.
Finally, the history of Kosteletzkya in the New World was set into motion by a relatively recent dispersal to the New World of a B-genome-bearing Kosteletzkya buettneri ancestor, followed by a rapid radiation to yield the seven known diploids in that hemisphere. A similar pattern, in which one among several African genomes is also found in the New-World, can be seen in Gossypium (
In reporting on chromosome numbers in Kosteletzkya, I used the numbers evidence to suggest that Africa was the birthplace of the genus (
Within the diversity of the New-World Kosteletzkya species there is little likelihood that any as-yet-undiscovered polyploids exist. Any interspecific New-World hybrid that formed would contain two B genomes, and in the event of a chromosome doubling, there would be four closely similar sets of chromosomes entering prophase I of meiosis. The result, barring strong preferential pairing, would be multivalent associations, confused and uneven segregation at anaphase, and consequently much reduced fertility.
An extension of this idea may explain why genome A, rather than one of the other two African genomes, is the one that is shared among the tetraploids Kosteletzkya begoniifolia, Kosteletzkya borkouana and Kosteletzkya rotundalata. While there is some disagreement about whether or not allopolyploidy occurs more commonly in hybrids between parents with a greater genetic distance between them (
The suggestion that a Kosteletzkya carrying a B genome made its pre-Columbian way across the Atlantic calls for an evaluation of the dispersal capabilities of the group.
My experience with Kosteletzkya indicates that both Mattei and Hochreutiner were correct. Adjacent capsule-valves in Kosteletzkya often do cohere in pairs or threes and separate together from the fruiting axis, carrying one or two seeds with them (Blanchard in
Kosteletzkya pentacarpos has been shown (as Kosteletzkya virginica [Linnaeus, 1753] A. Gray, 1849) to have an air space within the seed that permits it to float (
Dispersal over considerable distances appears to have been accomplished by several Kosteletzkya species. Known contemporary distributions suggest that Kosteletzkya adoensis has jumped from the African mainland to Madagascar, which is a minimum of 800 miles from the nearest known mainland population. It also appears to have dispersed westward from its main center in East Africa to the mountains of Cameroon (1100 miles) and the mountains of Sierra Leone (a further 1350 miles), with no known occurrences of the species—and no montane habitats—in the intervening areas. Kosteletzkya begoniifolia has made a similar jump from montane East Africa to Cameroon (1050 miles), and Kosteletzkya borkouana has dispersed from eastern Democratic Republic of the Congo and East Africa 1300 miles across a considerable expanse of the Sahara to the Borkou region of northern Chad (
It is worth noting, however, that some of these dispersal feats may have been aided in Africa by paleoclimatic cycles that provided geographically more benign intervening conditions (
Two Kosteletzkya species were unavailable for inclusion in the hybridization experiments reported here: Kosteletzkya thurberi from northwestern Mexico and the rare Kosteletzkya batacensis, endemic to the Philippine island of Luzon.It can be reasonably predicted that Kosteletzkya thurberi is a diploid of genomic makeup BB like all other New-World Kosteletzkya species. Kosteletzkya batacensis, on the other hand, remains a complete mystery. On the basis of general morphology—particularly of the fruits—the plant seems to belong in Kosteletzkya, although it is unique in the genus in being an annual. However its restricted range and its remote geographical location in relation to the rest of the genus would make any further speculation on its relationships almost reckless. There was an active maritime trade between Manila and Mexico for centuries, but suggestions of a Mexican origin for the plant (
In the two centers of diversity of Kosteletzkya, Africa and the northern Neotropics, the constituent species occur in approximately equal numbers and display similar ranges of morphological diversity. The results of the present study suggest that this apparent symmetry hides profound underlying differences in the evolutionary histories of the two groups. Pairing relationships in interspecific hybrids appear to show that Kosteletzkya in Africa underwent an early diversification at the diploid level, followed by a rich and complex history of allopolyploidy. In dramatic contrast, Kosteletzkya made a late appearance in the New World, where it underwent a rapid diploid-level diversification. These observations, especially if also borne out by a molecular study currently under way, lend strong support to my earlier contention, based on chromosome-number differences, that Africa is the birthplace of the genus. This scenario necessitates a long-distance dispersal, and the fruit and seed adaptations in Kosteletzkya, as well as known within-hemisphere dispersals, suggest such a capability.These observations also lend further weight to similar dispersals proposed by other workers to explain distributions in other malvoid genera, including the precursors of the cultivated cottons.
This manuscript has benefited from thoughtful comments by two anonymous reviewers. I am grateful to P. A. Fryxell, D. M. Bates, W. L. Handlos, C. H. S. Kabuye, and B. O. Daramola for providing viable seeds that were used in this study. I thank K. M. Neubig and N. H. Williams for their help in the preparation of the plates and diagrams for the paper’s figures, and K. M. Neubig for providing most of the flower photographs. W. S. Judd, K. M. Neubig and J. H. Blanchard read and commented helpfully on an earlier draft of this manuscript. I am grateful to M. Levy, then Director of the Kriebel Herbarium at Purdue University (PUL) and N. H. Williams, Keeper of the University of Florida Herbarium (FLAS), for their hospitality during my pursuit of some of this work. Funds supporting some of the research described here came from Faculty Development Grants from Earlham College and Research Grants from the Research Committee of Long Island University/C. W. Post Campus, as well as from personal resources.