(C) 2011 SoniaJ. R. Proença. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The ribosomal clusters of six Paleartic taxa belonging to the tiger beetle genera Cephalota Dokhtourow, 1883and Cylindera Westwood, 1831, with multiple sex chromosomes (XXY, XXXY and XXXXY) have been localised on mitotic and meiotic cells by fluorescence in situ hybridization (FISH), using a PCR-amplified 18S rDNA fragment as a probe. Four patterns of rDNA localization in these tiger beetles were found: 1. Two clusters located in one autosomal pair; 2. Two clusters located in one autosomal pair and one in an X chromosome; 3. Three clusters located in three heterosomes (XXY); 4. Two clusters located in one autosomal pair and two in the heterosomes (one of the Xs and the Y). These results illustrate that ribosomal cistrons have changed their number and localization during the evolution of these genera, showing a dynamic rather than a conservative pattern. These changes in rDNA localization are uncoupled with changes in the number of autosomes and/or heterosomes. A mechanism that involves transposable elements that carry ribosomal cistrons appears to be the most plausible explanation for these dynamics that involve jumping from one location in the genome to another, in some cases leaving copies in the original location.
Cephalota, Cylindera, Cicindelini, Coleoptera, FISH, ribosomal genes, chromosome evolution
Often closely related species differ in their karyotypes,
both in terms of changes in chromosome number and morphology and/or
localization of genes in chromosomes. Whether these changes have played a
significant role as isolation mechanisms in speciation (
Characterization of the number and distribution of ribosomal DNA (rDNA) genes using fluorescence in situ
hybridization (FISH) provides landmarks for the construction of
physical maps in comparative genomics, and is useful for phylogenetic
and evolutionary studies.
These interspecific differences are mirrored, as
expected, by intraspecific differences in particular species of tiger
beetles. A population study regarding the number and localization of
rDNA clusters in Cicindela (Calomera) littoralis and Lophyra flexuosa
showed that both species were polymorphic for these traits as a single
population of each species had an rDNA localization different from all
the other populations (
In this paper we apply fluorescence in situ hybridization with a 18S ribosomal probe to six Paleartic taxa of the genera Cephalota and Cylindera with different sex chromosome systems (XO, XXY, XXXY, XXXXY), some of which were previously studied cytogenetically by
Individuals belonging to the six species studied were collected in the localities listed in Table 1. Males and females were analysed in all species, although in Cephalota deserticoloides, Cephalota circumdata and Cylindera paludosa, only males provided interpretable plates.
The specimens were identified by the authors and are deposited in the collection of the Department of Animal Biology, University of Lisbon, and in the Department of Zoology and Physical Anhropology, University of Murcia.
Chromosome preparationsKaryological analyses were carried out on gonads dissected from beetles anaesthetised with ethyl-acetate. Testes and ovaries were given a hypotonic treatment in distilled water and fixed using fresh ethanol-acetic acid solution (3:1) for 1 h, with several changes of the fixative solution during the next day and were kept at –20 ºC until studied. Squashes were made on a slide in 70% acetic acid and coverslips were removed after freezing in liquid nitrogen. The slides containing well spread mitotic and meiotic figures were aged for at least 3 days in a 37 ºC incubator.
In situ hybridizationFISH was performed as previously described (
Active NORs were detected with silver according to the Howell and Black (1980) technique, with slight modifications. Two solutions were prepared, one colloidal developer containing 0.2 g powdered gelatine in 10 ml distilled water and 0, 1 ml of formic acid and a solution of 50% AgNO3, centrifuged at 13000 g for 5 min to separate the silver previously precipitated and kept in the dark. One part of the colloidal developer and two parts of the silver solution were placed on the slides, mixed, covered with a coverslip and incubated at 70 ºC on a hot plate until the solution has turned a deep golden-brown colour. The slides were rinsed thoroughly in distilled water, counterstained with 5% Giemsa in phosphate buffer pH 6.8, washed and air-dried.
ResultsDetailed karyotypes of the six species investigated have been reported previously (
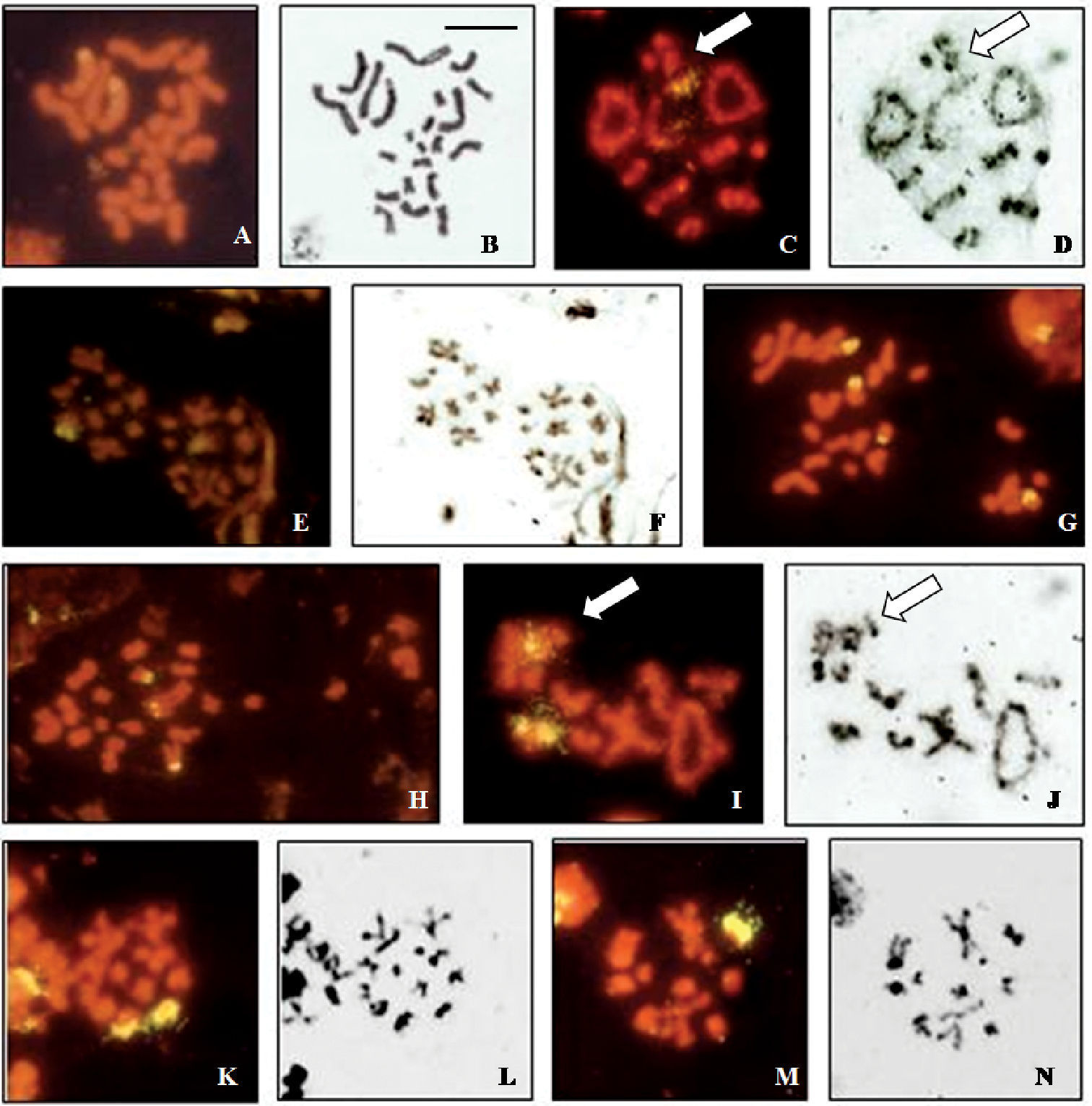
The rDNA probe hybridizes to the third autosomal pair in male and female mitotic figures of Cephalota hispanica (Fig. 2a, b) and the signal is distantly located. Meiotic figures confirm hybridization on the third autosomal bivalent (Fig. 2c, d). Second metaphase plates have one signal each, 9+X1X2 and 9+Y (Fig. 2e, f).
Cephalota maura shows four signals in female mitotic metaphases (Fig. 2g), in two small and two medium-sized chromosomes. Male mitotic plates show signals in two small and one medium-sized element (Fig. 2h). In male diakinesis two fluorescent signals are seen in one small autosomal bivalent and one signal in the sex vesicle (Fig. 2i, j), most likely in one of the Xs. This is confirmed by the observation of second metaphase plates that are of two types, with 12 elements (9+X1X2X3, Fig. 2k, l) and 2 signals, and with 10 elements (9+Y, Fig. 2m, n) and one signal.
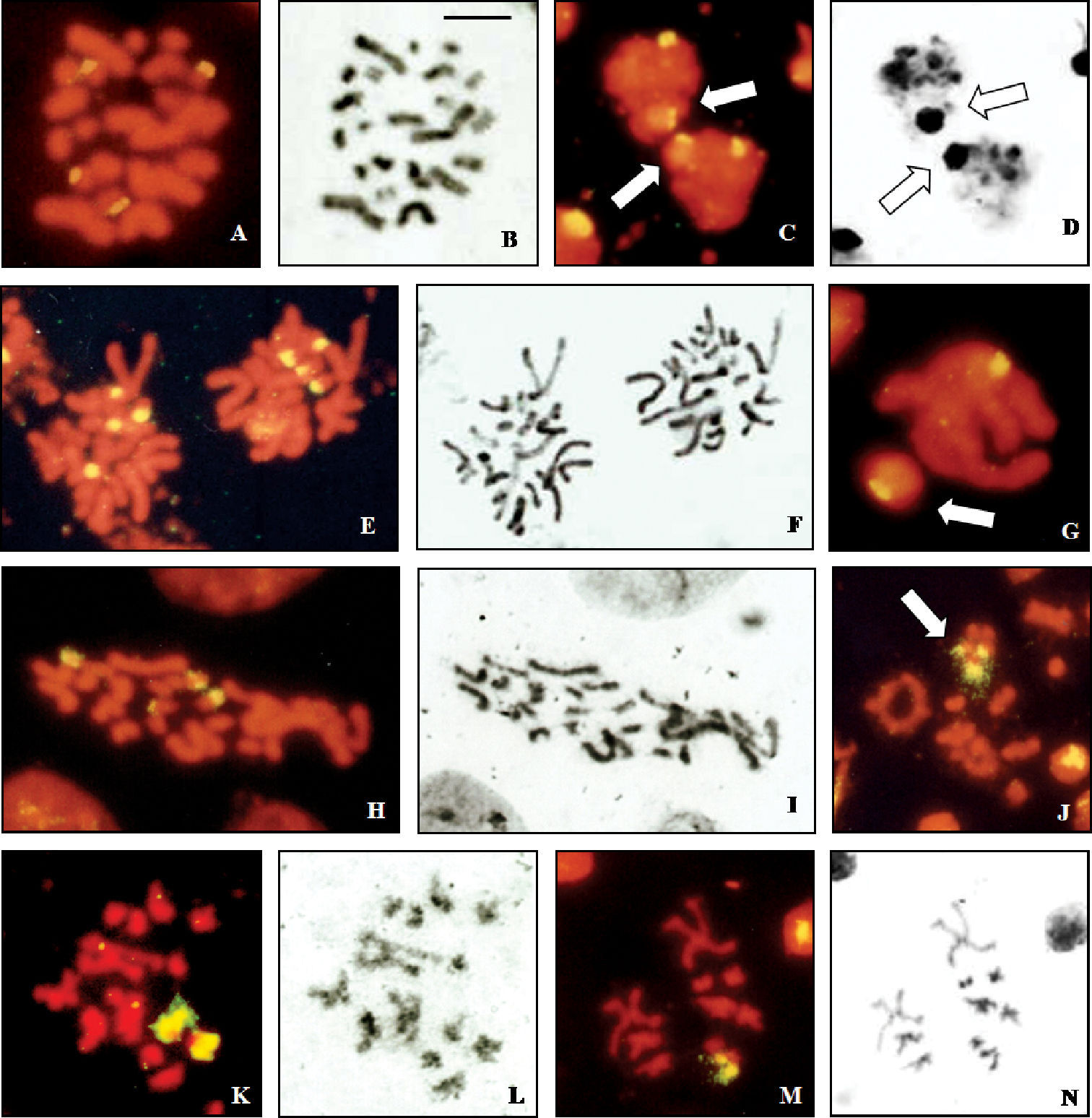
Male spermatogonial mitosis of Cephalota deserticoloides shows signals in four small chromosomes (Fig. 3a, b). Meiotic plates show two hybridization sites in the sex vesicle and one additional site in one autosomal bivalent (Fig. 3 c, d). A similar pattern is shown by Cephalota circumdata, which has signals in 4 chromosomes in male mitosis (Fig. 3e, f). First metaphase plates of this species have one autosomal bivalent and two heterosomes labelled (Fig. 3g).
A different situation is present in Cylindera trisignata, where three labelled chromosomes are observed in male mitosis. These chromosomes may correspond to 3 of the 5 heterosomes as they are of different (from medium to small) size. Female mitotic plates show four signals in one small and one medium-sized pairs (Fig. 3h, i). Male meiotic figures confirm this interpretation and show fluorescent signal in three of the five elements of the sex vesicle (Fig. 3j). This is further confirmed in second metaphase plates where two types are observed. Two signals are present in cells with 9+X1X2X3X4 (Fig. 3k, l) and one signal is present in cells with 9+Y (Fig. 3m, n).
Cylindera paludosa
is the only species studied without multiple sex chromosomes (males are
n= 7+XO). Observations here made in individuals from Salinas de Pinilla
agree with the description of the karyotype (
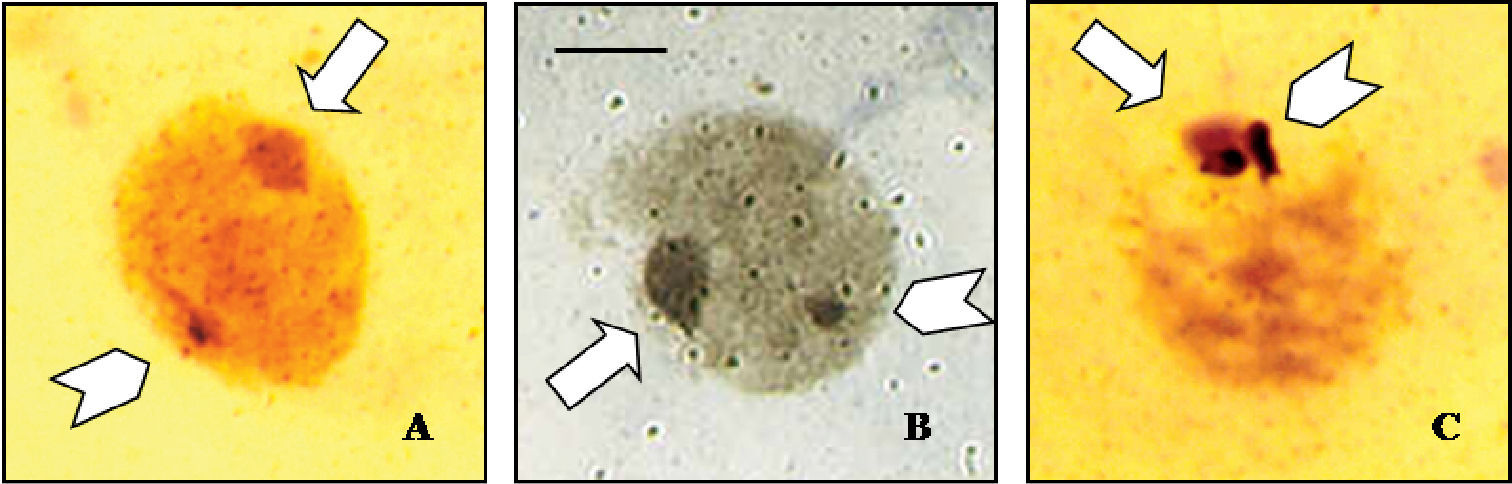
Silver staining was performed for Cephalota hispanica, Cephalota circumdata and Cephalota trisignata to locate active NORs in interphase nuclei. Cephalota hispanica and Cephalota circumdata showed silver precipitates outside the condensed sex vesicle in interphase nuclei (Fig. 4 a, b), and Cephalota trisignata showed silver precipitates inside the sex vesicle (Fig. 4c).
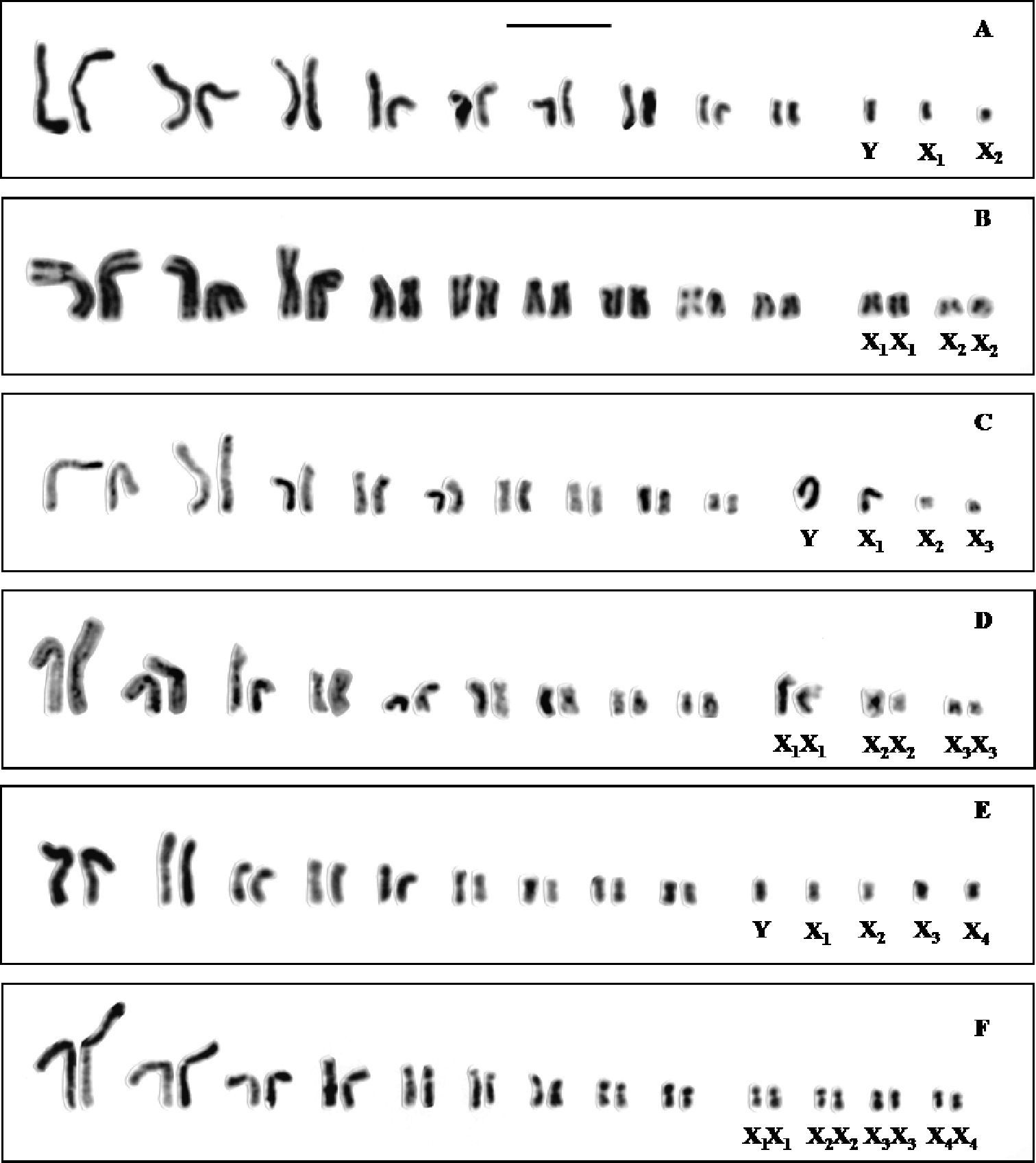
Standard karyotypesof a Cephalota hispanica male b Cephalota hispanica female c Cephalota maura male d Cephalota maura female e Cephalota trisignata male and f Cephalota trisignata female. Bar = 5µm.
Localities, male meioformula and pattern of rDNA localization for sampled species of tiger beetles.
| Species | Localities | Meioformula | Pattern of rDNA localisation |
|---|---|---|---|
| Cephalota (Cassolaia) maura (Linnaeus 1758) | Castro Marim (Portugal) | 9 + XXXY | (3 signals) Autosomes (2), Heterosome (X) |
| Cephalota (Cephalota) hispanica (Gory 1833) | Castro Marim (Portugal) | 9 + XXY | (2 signals) Autosomes |
| Cephalota (Taenidia) circumdata imperialis (Klug 1834) | Salinas de Pinilla (Spain) | 9 + XXXY | (4 signals) Autosomes (2), Heterosome (XY) |
| Cephalota (Taenidia) deserticoloides (Codina 1931) | Albatera (Spain) | 9 + XXXY | (4 signals) Autosomes (2), Heterosome (XY) |
| Cylindera (Cylindera) paludosa (Dufour 1820) | Salinas de Pinilla (Spain) | 7 + X0 | (2 signals) Autosomes |
| Cylindera (Eugrapha) trisignata (Dejean 1822) | Carrapateira (Portugal) | 9 + XXXXY | (3 signals) Heterosomes (XXY) |
The results for Cylindera paludosa corroborate previous findings (
Four patterns of rDNA localization were found in the
tiger beetles species analysed in this paper. These patterns are: i) One
cluster located in each member of an autosomal pair (two signals); ii)
Two clusters located in an autosomal pair and one in an X chromosome
(three signals); iii) Three clusters located in three of the heterosomes
XXY (three signals); and iv) One cluster located in each member of an
autosomal pair and in two of the heterosomes, apparently one of the Xs
and the Y (four signals). The last two patterns are described for the
first time, and are added to the four previously described in the genus
Cicindela and related taxa by
Species of the more primitive lineages such as Amblycheila, Megacephala and Mantichora have from two to four autosomal pairs carrying ribosomal genes (
The results in Cylindera trisignata,
(X1X2X3X4Y) give some clues about the origin of the fourth X. This
species has three heterosomes with ribosomal genes. This fact may
support the hypothesis of a mechanism of X dissociation rather than the
incorporation of autosomal segments into the multiple sex chromosomes
system as the origin of the 4X condition. This last hypothesis was
proposed by
Localization of rDNA clusters in Cicindela, revealed by in situ hybridization of the PCR amplified ribosomal probe to squashed mitotic and meiotic chromosomes. The phase contrast image of some cells is figured to the right of the fluorescence image. a, b Cephalota hispanica, female mitotic metaphase c, d Cephalota hispanica, male metaphase I plate; n=9+X1X2Y, e, f Cephalota hispanica, male metaphase II plates, n = 9+Y and n = 9+X1X2, g Cephalota maura, female mitotic metaphase h Cephalota maura, male mitotic metaphase i, j Cephalota maura, male metaphase I plate; n=9+X1X2X3Y k, l Cephalota maura, male metaphase II plate (n=9+ X1X2X3) m, n Cephalota maura, metaphase II plate (n=9+Y). Arrows indicate the sex chromatin. Bar = 5µm.
Localization of rDNA clusters in Cicindela, revealed by in situ hybridization of the PCR amplified ribosomal probe to squashed mitotic and meiotic chromosomes. The phase contrast image of some cells is figured to the right of the fluorescence image. a, b Cephalota deserticoloides, male mitotic metaphase c, d Cephalota deserticoloides, zygotene nuclei; n=9+X1X2X3Y, e, f Cephalota circumdata, male mitotic metaphase g Cephalota circumdata, zygotene nuclei; n=9+X1X2X3Y h, i Cephalota trisignata, female mitotic metaphase j Cylindera trisignata, metaphase I plate, n=9+X1X2X3X4Y k, l Cylindera trisignata, male metaphase II plate (n=9+ X1X2X3X4) m, n Cylindera trisignata, male metaphase II plate (n=9+Y). Arrows indicate the sex chromatin. Bar = 5 µm.
Silver staining of meiotic nuclei, showing nucleolar activity on the autosomes of a Cephalota hispanica, and b Cephalota circumdata, and on the sex chromosomes of c Cylindera trisignata. Arrows indicate the sex chromatin and arrowheads point the nucleolar active sites. Bar = 5 µm.
This work has been supported by Fundação para a Ciência e Tecnologia, PRAXIS XXI/BD/15986/98, Portugal and Project No CLG2008-03628 of the DGI of the Spanish Ministry of Science and Innovation. Thanks are due to Elena Martínez-Navarro for technical assistance, and José Fermín Sánchez-Gea and Pilar De la Rúa for useful comments and discussion. Prof. Bárbara Fernández is acknowledged for facilitating the use of the fluorescence microscope.