(C) 2011 Lorena Corina Bezerra de Lima. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Tetraodontiformes are the most derived group of teleostean fish. Among other apomorphies, they are characterized by a high degree of fusions or significant bone loss in the head and body. In the early phylogenetic proposals presented for this order, the families Balistidae and Monacanthidae have been unanimously considered to be closely related. Although they have moderate species diversity, they are scarcely known in cytogenetic aspect and chromosomal pattern comparisons between these groups have yet to be established. The species Cantherhines macrocerus (Hollard, 1853), Cantherhines pullus (Ranzani, 1842) (Monacanthidae) and Melichthys niger (Bloch, 1786) (Balistidae) were cytogenetically analyzed using conventional (Ag-impregnation, C-banding, CMA3- and DAPI-fluorescence) and molecular (FISH with an 18S rDNA probe) cytogenetic protocols. The karyotypes of all three species were very similar possessing diploid chromosome numbers 2n = 40 and composed exclusively of acrocentric chromosomes. Single NOR-bearing pair as well as positive heterochromatic blocks at pericentromeric regions were identified in the karyotypes of the three species studied. NOR-bearing sites were positively labeled after Ag-impregnation, C-banding, CMA3-fluorescence and FISH with an 18S rDNA probe but were negative after DAPI-fluorescence. Such remarkable shared conspicuous chromosomal characters corroborate either close phylogenetic relationship of these families, previously established by morphological and molecular data, or rather conservative nature of karyotype differentiation processes. The later hypothesis, however, appears less probable due to centric or in tandem fusions documented for another Balistoidea species.
Balistoidea, fish cytogenetics, karyotype evolution, Tetraodontiformes
The order Tetraodontiformes, which stands out among
marine fish for its marked diversity, is composed of approximately 430
species distributed in nine families (
Among the Tetraodontiformes, the superfamily Balistoidea (leatherjackets) includes the families Balistidae (triggerfish) and Monacanthidae (filefish), with a fossil record that dates back to the Early Eocene and probably to the Late Cretaceous (
To date nearly 60 species of Tetraodontiformes have been cytogenetically studied (
In this work, we revise cytogenetic data for Melichthys niger (Bloch, 1786) (Balistidae) and describe the karyotype and other chromosomal characteristics for Cantherhines macrocerus (Hollard, 1853) and Cantherhines pullus (Ranzani, 1842) (Monacanthidae), to compare the chromosomal patterns of the families Balistidae and Monacanthidae, estimating their divergence level.
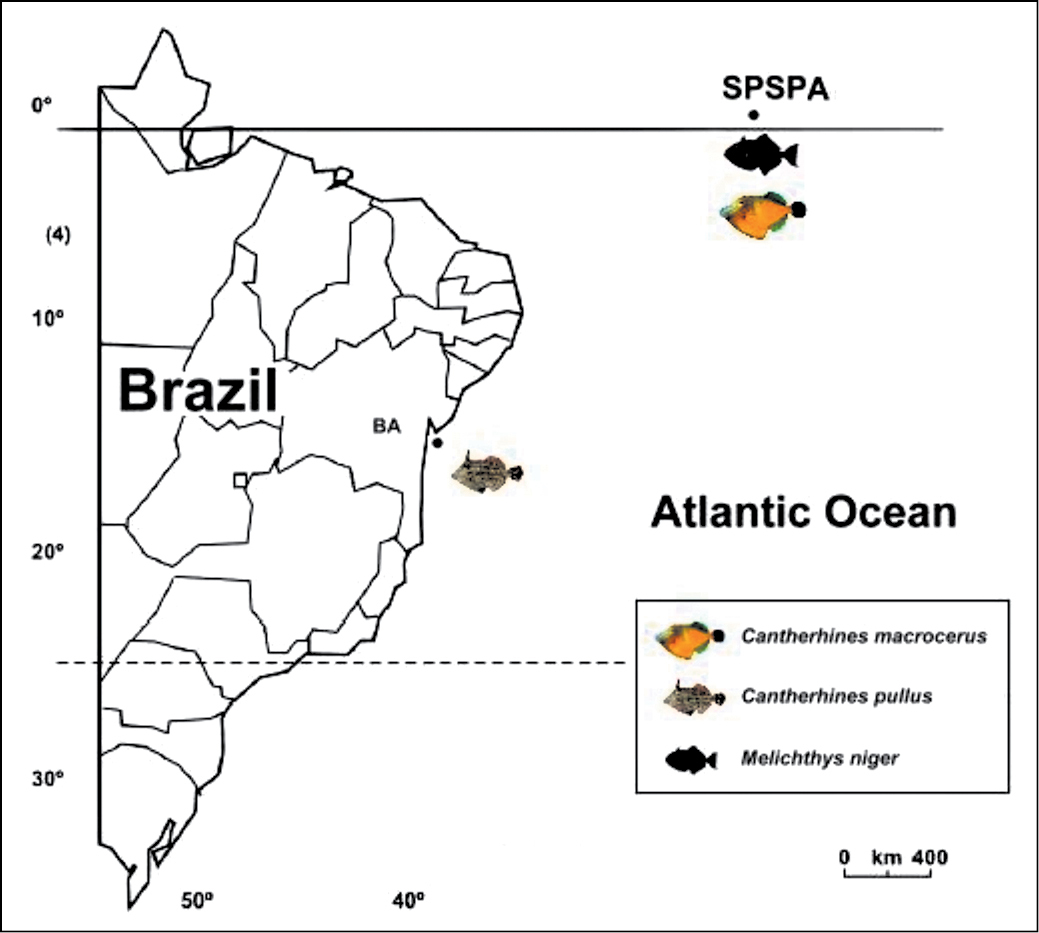
Material and methodsWe analyzed 42 specimens of Melichthys niger and 14 of Cantherhines macrocerus, collected in the Saint Peter and Saint Paul’s Archipelago (00º55’15” N, 029º20’60” W), 1010 km from the Brazilian northeastern coast and about 1.824 km) from the African coast, and two specimens of Cantherhines pullus collected in the coastal region of Salvador (12º58’S, 38º31’W), Bahia state, northeastern Brazil (Fig. 1).
Figure 1. Map showing the geographic collection points of the species Melichthys niger, Cantherhines macrocerus and Cantherhines pullus. SPSPA – Saint Peter and Saint Paul’s Archipelago; BA – Bahia state.
The specimens were subjected to mitotic stimulation as proposed by
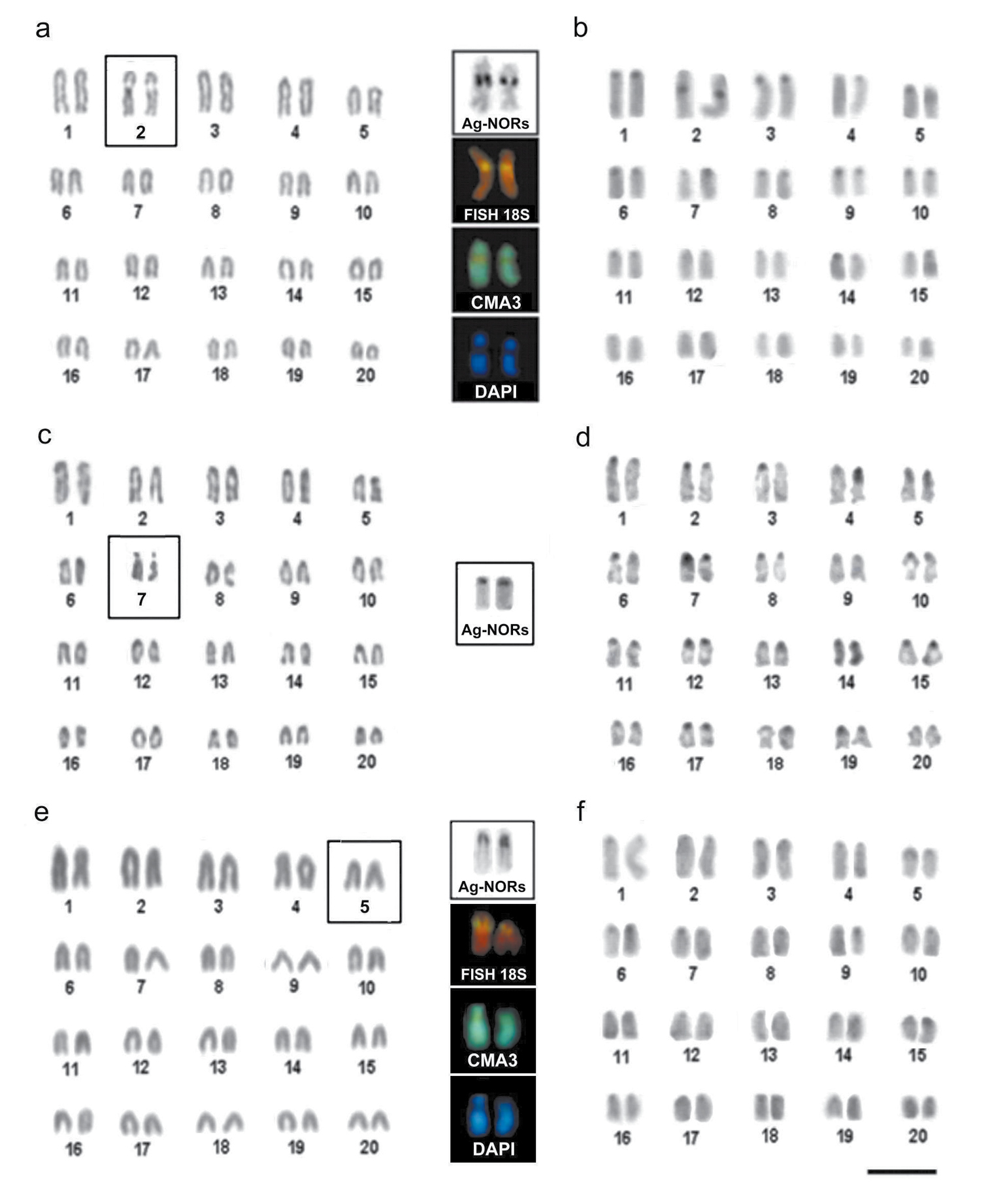
The specimens of Melichthys niger showed 2n=40 chromosomes and a karyotype consisting of 20 pairs of acrocentric (a) chromosomes (NF=40) (Fig. 2a). The presence of a conspicuous secondary constriction was observed in the interstitial position on the long arm of the chromosome pair No. 2, corresponding to the nucleolus organizer regions (NORs), identified by Ag-NOR sites and by in situ hybridization with an 18S rDNA ribosomal probes (Fig. 2, upper boxes). The heterochromatic blocks were reduced in size and dispersed in the pericentromeric regions in most of the chromosome pairs (Fig. 2b). The NORs were heterochromatic and CMA3 positive and DAPI negative (Fig. 2, upper boxes).
Figure 2. Karyotypes of Melichthys niger (a, b), Cantherhines macrocerus (c, d) and Cantherhines pullus (e, f), arranged from Giemsa stained (a, c, e) and C-banded chromosomes (b, d, f). In the center highlighted are the NOR-bearing pairs of analyzed species (2nd, 7th and 5th, respectively) after Ag-NOR staining, in situ hybridization with an 18S rDNA probe, CMA3 and DAPI fluorescence. Bar = 5µm.
The specimens of Cantherhines macrocerus (Monacanthidae) had 2n=40 chromosomes and karyotype composed of a acrocentric chromosomes (Fig. 2c). Ag-NOR sites were located in the pair No. 7 in interstitial region, near the centromere (Figure 2, middle box). C-banding revealed heterochromatic blocks distributed in the pericentromeric region in most of the chromosome pairs (Figure 2d) and more intensively stained on the secondary constriction of the NOR-bearing pair. In this species, experiments using FISH probes and fluorochrome staining were unsuccessful.
The specimens of Cantherhines pullus showed 2n=40 chromosomes and karyotype composed entirely of acrocentric chromosomes (Fig. 2e). The NORs were identified at the pericentromeric position of pair No. 5, as revealed by Ag-NOR-staining and FISH with an 18S rDNA probes (Fig. 2, lower box). The heterochromatic regions were distributed in centrometric and pericentromeric positions in most of the chromosomes. The NORs sites were heterochromatic (Fig. 2f), and CMA positive and DAPI negative (Fig. 2, lower box). None of the karyotypes displayed sex-related chromosome heteromorphism.
DiscussionSix out of the ten karyotyped species in the family Monacanthidae have diploid numbers ranging from 2n = 33/34 to 36 chromosomes. Such low 2n numbers have been a noticeable characteristic for Monacanthidae species. The present data for Cantherhines macrocerus and Cantherhines pullus increase the range of the highest 2n for representatives of this family. Surveys involving a larger number of genera may confirm a possible basal karyotype with 40 chromosomes for this family, showing on average lower diploid values than those of the Balistidae. Based on chromosomal number, Cantherhines macrocerus and Cantherhines pullus would be placed in the family Balistidae.
The karyotype of the individuals of Cantherhines macrocerus
from the Saint Peter and Saint Paul’s Archipelago was similar to those
described for specimens from the coast of Rio de Janeiro (
In this study, the data obtained for Melichthys niger corroborate results described for the species earlier (
The chromosomal characteristics observed in Cantherhines macrocerus, Cantherhines pullus and Melichthys niger, like the presence of single NOR and the pericentromeric heterochromatin blocks as reported in other Tetraodontiformes (
In the karyotypes of species under our study, as well as in other representatives of Balistidae and Monacanthidae (
Repetitive sequences and transposition elements are
closely related to the heterochromatic regions, and although account
for less than 10% of the genome of the Tetraodontiformes studied (
Among the Perciformes, the presence of single interstitial NORs is common, representing an ancestral condition (
The set of cytogenetic characters already available in Monacanthidae and Balistidae
species indicate a greater karyotypic similarities and common
tendencies of karyotype evolution with other groups of the order
Tetraodontiformes, corroborating previous analyses based on
morphological and molecular data (
We would like to thank Universidade Federal do Rio Grande do Norte (UFRN) and Comissão Interministerial para os Recursos do Mar (CIRM) for providing the facilities and proper conditions for the accomplishment of the present survey and José Garcia Jr for species identification. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Proc. 556793/2009-9).