(C) 2011 Puneet Kumar. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In this study, we examined the chromosome number, detailed male meiosis, microsporogenesis, pollen fertility and morphological features and distribution of 2× and 4× cytotypes of Ranunculus hirtellus Royle, 1834. The majority of the populations scored now from cold regions of the northwest Himalayas showed tetraploid (n=16) meiotic chromosome count and one of the populations studied from the Manimahesh hills existed at diploid level (n=8). The individuals of diploid cytotype exhibited perfectly normal meiotic course resulting in 100% pollen fertility and pollen grains of uniform sizes. On the other hand, the plants of the tetraploid cytotype from all the populations in spite of showing normal bivalent formation and equal distribution to the opposite poles at anaphases showed various meiotic abnormalities. The most prominent among these meiotic abnormalities was the cytomixis which involved inter PMC (pollen mother cell) chromatin material transfer at different stages of meiosis-I. The phenomenon of cytomixis induced various meiotic abnormalities which include chromatin stickiness, pycnotic chromatin, laggards and chromatin bridges, out of plate bivalents at metaphase-I, disoriented chromatin material at anaphase/telophase and micronuclei. Consequently, these populations exhibited varying percentages of pollen sterility (24 - 77 %) and pollen grains of heterogeneous sizes. Analysis of various morphometric features including the stomata in 2× and 4× cytotypes showed that increase in ploidy level in the species is correlated with gigantism of vegetative and floral characters and the two cytotypes can be distinguished from each other on the basis of morphological characters. The distribution patterns of the 2× and 4× cytotypes now detected and 2×, 3×, 4× cytotypes detected earlier by workers from other regions of the Indian Himalayas have also been discussed.
chromosome number, cytotype, cytomixis, Lahaul-Spiti, Manali hills, Manimahesh hills, meiotic abnormalities, stomata
Ranunculus hirtellus Royle, 1834 (Ranunculaceae),
a perennial erect or decumbent herb, distinctly pubescent with
fibrous and shortly fusiform root stock is endemic to Himalayas, and
distributed in the temperate, sub-alpine and alpine slopes in
North-West to North-East Himalaya, temperate to subalpine slopes at
2000 – 4500 m in the states of Jammu & Kashmir, Himachal Pradesh,
Uttarakhand, Sikkim, and Arunachal Pradesh and also in Afghanistan,
Pakistan, Nepal and Tibet (
The species is highly variable with respect to habit,
plant size, shape and hairiness of leaves and sepals, hairiness of
pedicels, and size of flowers (
Our research group has been engaged in the study of
cytological aspects of plants of cold deserts of India through male
meiosis since 2006. Male meiosis of more than 300 species from the cold
desert areas of Chamba, Lahaul-Spiti and Kinnaur districts of Himachal
Pradesh has been studied and various aberrations were detected during
male meiosis in Caltha palustris Linnaeus, 1753(
The aim of the present research was to study the male meiosis in detail and to find the impact of chromatin transfer in inducing meiotic aberrations and their consequent effect on pollen fertility and pollen size. The purpose of the present study was also to differentiate the 2× and 4× individuals growing wild and also to find out the distribution patterns of different cytotypes in the Indian Himalayas.
Material and methodsPlant material and identification– Material for
male meiotic studies were collected from the wild plants growing in
different localities of Lahaul-Spiti, Manimahesh hills and Manali hills
of Himachal Pradesh, India in the months of May - July during the
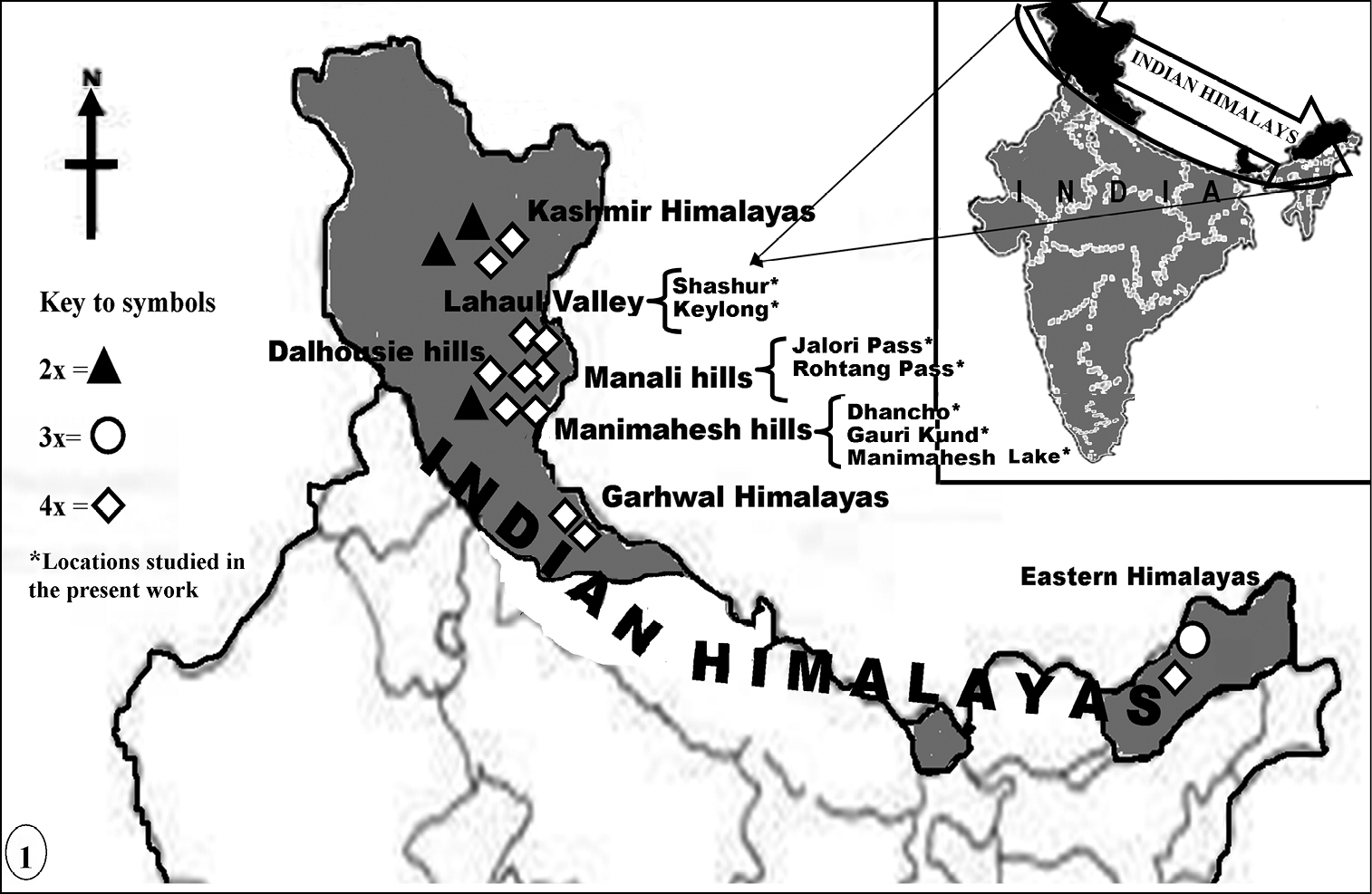
years 2008 and 2009 (Table 1, Fig. 1). The identification of the taxon was done by consulting the various floras of the region such as, Flora of Lahaul-Spiti (
List of specimen number/s, meiotic chromosome
number, and places of collection with district, province, habitat,
latitude and longitude, altitude and habitat of different populations
of the diploid (2n = 2× = 16) and tetraploid (2n = 4× = 32) cytotypes of Ranunculus hirtellus. *Herbarium code as per “Index Herbariorum” by
| Cytotype | Specimen number (PUN*) | Meiotic chromosome number (n) | Places of collection with district, province, habitat, latitude and longitude and altitude in meters (Alt. m) |
| Diploid | 51801 | 8 | Gauri Kund, Manimahesh hills, Chamba, Himachal Pradesh, alpine moist slopes, 32°24.11'N; 76°38.25'E , Alt.: 3930 m |
| Tetraploid | 51370 | 16 | Manimahesh Lake, Manimahesh hills, Chamba, Himachal Pradesh, alpine moist slopes, 32°23.91'N; 76°38.30'E , Alt.: 4300 m |
| 51356 | 16 | Dhancho, Manimahesh hills, Chamba, Himachal Pradesh, along water course, 32°25.18'N; 76°36.53'E , Alt.: 3030 m | |
| 51360 | 16 | Jalori Pass, Manali hills in Kullu, Himachal Pradesh, moist slopes in Oak forest, 31°31.95'N; 77°23.87'E , Alt.: 3140 m | |
| 51364 | 16 | Rohtang Pass, Manali hills in Kullu, Himachal Pradesh, alpine moist slopes, 32°21.84'N; 77°14.59'E , Alt.: 3980 m | |
| 51138 | 16 | Shashur, Lahaul Valley in Lahaul-Spiti, Himachal Pradesh, open and moist grassy slopes among scattered trees of Salix and Juniperus 32°34.56'N; 77°1.54'E , Alt.: 3340 m | |
| 51374 | 16 | Keylong, Lahaul Valley in Lahaul-Spiti, Himachal Pradesh, growing under Salix trees in moist conditions, 32°34.18'N; 77°2.01'E , Alt.: 3340 m |
Map showing the distribution pattern of the 2× and 4× cytotypes reported here (marked with asterisks) and the 2×, 3×, 4× cytotypes detected by workers from other regions of the Indian Himalayas.
Chromosome counts and male meiotic analysis– Developing anthers from floral buds were squashed in 1% acetocarmine and preparations were studied for chromosome counts, and detailed meiotic behavior in pollen mother cells (PMCs) at early prophase-I, metaphase-I (MI), anaphases-I/II (AI/II), telophases-I/II (TI/II) and sporad stage. In populations with normal meiotic course, a total of 10–30 PMCs were examined for determining the chromosome counts while in cytologically abnormal populations 20–50 slides prepared from different anthers/flowers (with 100–200 PMCs) were analyzed in each case.
Pollen fertility– Pollen fertility was estimated through stainability tests for which anthers of mature flowers were squashed in glyceroacetocarmine mixture (1:1) or 1% aniline blue dye. 200–500 pollen grains were analyzed in each case for pollen fertility and pollen size. Well-filled pollen grains with uniformly darkly stained cytoplasm were scored as fertile/viable while shrivelled pollen with unstained or poorly stained cytoplasm were counted as sterile/unviable. Pollen fertility was expressed as an average percentage of the stained pollen grains/total pollen grains analyzed. Size of stained pollen grains was measured with occulomicrometer.
Photomicrographs– Chromosome spreads were analyzed with Olympus light microscope and the best plates of chromosome counts, meiotic abnormalities, sporads and pollen grains (fertile, sterile) were photographed from the temporary mounts with Nikon Eclipse 80i microscope.
ResultsRanunculus hirtellus has been worked out for male meiosis and morphometric analysis from seven different localities of Manimahesh and Manali hills, and Lahaul-Spiti (Table 1). Two intraspecific cytotypes (Fig. 2, A & B), the diploid (n=8) and the tetraploid (n=16) have been detected in the species. The population scored from the Manimahesh hills was found to be diploid while rest of the six populations studied from Kullu, Chamba and Lahaul-Spiti districts existed at tetraploid level. Cytological and morphometric analysis have been performed on both the cytotypes and data regarding the micro- and macroscopic characters are provided in Table 2.
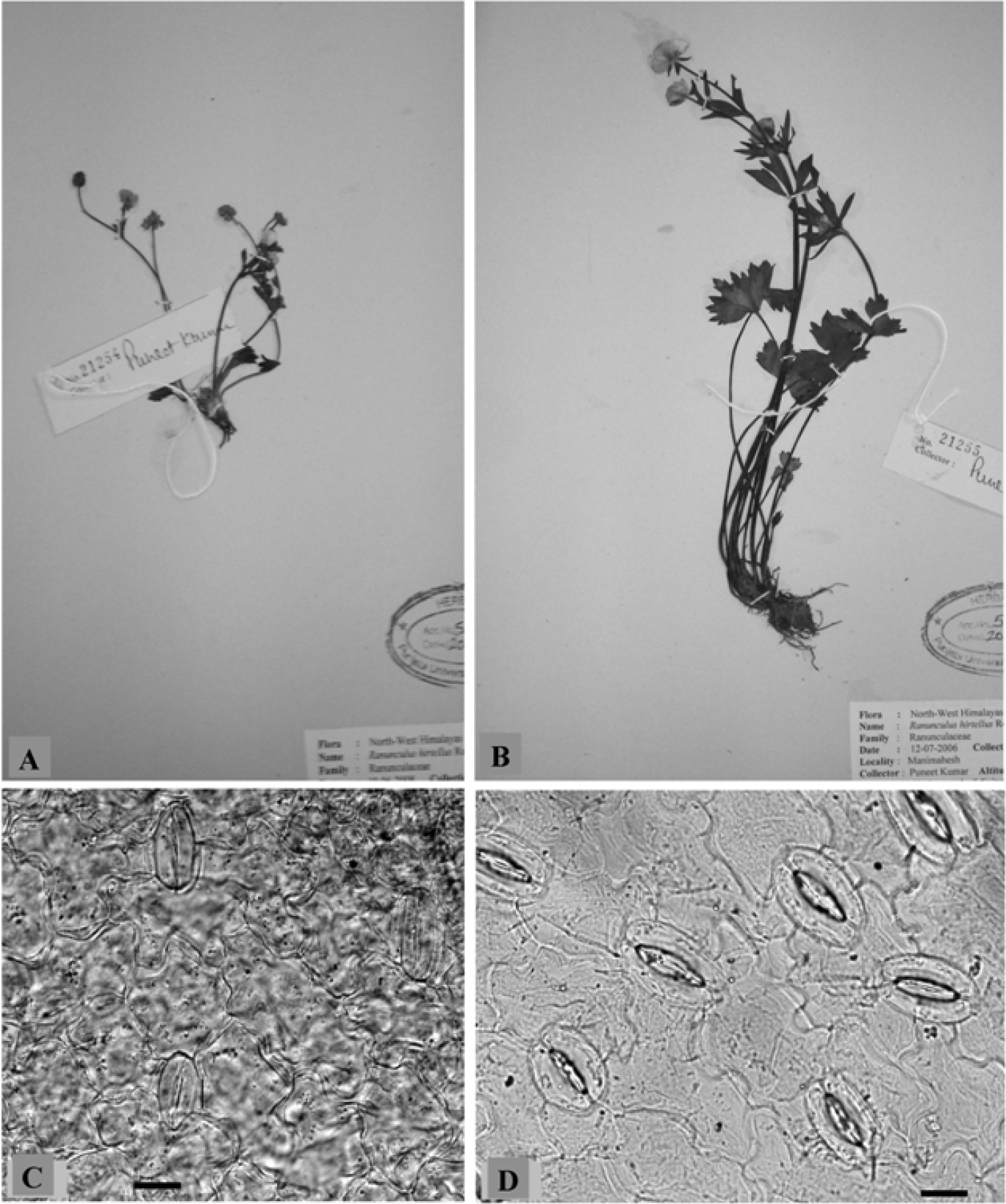
Individuals of Ranunculus hirtellus A 2× B 4× cytotype. Stomata C 2× and D 4× cytotype. Scale bars = 20 μm.
Comparison of micro- and macroscopic characters of the diploid (2n = 2× = 16) and tetraploid (2n = 4× = 32) cytotypes of Ranunculus hirtellus(Figures in the parentheses represent the mean ± standard deviation). *4× populations from Manimahesh Lake and Keylong
| S. No. | Characters | Cytotype | |
| Diploid | Tetraploid | ||
| 1. | Meiotic chromosome number (n) | 8 | 16 |
| 2. | Plant height (cm) | 21.20–23.50 (22.41±0.89) | 34.80–37.20 (35.78±0.90) |
| 3. | Radical leaf length (cm) | 6.80–10.28 (8.03±1.45) | 16.80–22.40 (18.58 ±2.71) |
| 4. | Number of flowers/plant | 15–21 (16 ±2.11) | 18–27 (25.3±2.7) |
| 5. | Stomatal size (µm) | 29.54–39.29 × 17.08–27.10 (34.04±2.47) (21.14±2.56) | 34.55–45.46 × 23.56–28.41 (39.15±3.23) (26.98±1.77) |
| 6. | Stomatal density/mm2 | 63–127 | 90–137 |
| 7. | Stomatal index | 11.47 | 24.94 |
| 8. | Pollen grain size (µm) | 24.52 - 24.85 × 25.13 - 26.55 (24. 63±0.35) (25.95±1.26) | 24.52 × 25.13* 24.85 × 26.55* |
Morphometric analysis involves both macro- and microscopic characters (Table 2). Macroscopic characters including plant height, radical leaf length and number of flowers per plant were studied from all the populations of the tetraploid and one population of the diploid cytotype. The tetraploid plants measured in height were much taller than the diploid. Also the radical leaves were noticed to be much larger in the tetraploid cytotype compared with the diploid. The number of flowers was more in the tetraploid compared to the diploid. Stomata were analysed from 2× population collected from Manimahesh Lake, 4300 mand the 4× population from Keylong, 3340 m (Fig. 2, C & D). The values for stomatal size, density and index were found to be more in the tetraploid compared to the diploid (Table 2). Pollen grains in the diploid cytotype were almost uniform sized whereas in the tetraploid cytotype pollen grains were of variable sizes except for two populations (Table 3).
Pollen grain size, relative frequency of variable sized pollen grains and pollen sterility in diploid 2× and tetraploid 4× cytotypes of Ranunculus hirtellus(Figures in the parentheses represent the mean ± standard deviation). Rf = relative frequency of variable sized pollen grains.
| S. No. | Populations | Pollen grains size (µm) | Rf % age | Pollen sterility % age | |
|---|---|---|---|---|---|
| Diploid | Tetraploid | ||||
| 1. | Gauri Kund | 24.52 - 24.85 × 25.13 - 26.55 (24. 63±0.35) (25.95±1.26) | 100 | 00 | |
| 2. | Dhancho | 59.96 × 59.9640.04 × 40.0432.76-36.40 × 29.12 - 36.40(35.25±4.21) (33.72±5.13)21.84 - 29.12 × 21.84 - 25.48(24.66±2.03) (23.66±1.97) | 3.5128.0735.0933.33 | 64 | |
| 3. | Manimahesh Lake | 24.52 × 25.13 | 100 | 26 | |
| 4. | Jalori Pass | 36.40-40.04 × 36.40(37.44±1.71)32.76 × 25.48-32.76(31.08±2.83)25.48 × 25.4821.84 × 21.8410.92 × 10.92 | 1.5839.1037.5020.631.19 | 77 | |
| 5. | Rohtang Pass | 19.27-23.85 × 20.64-27.52(21.73±1.43) (25.05±1.48)16.05-16.51 × 17.89 - 18.35(16.28±0.19) (18.05±0.31) | 53.9946.01 | 56 | |
| 6. | Keylong | 24.85 × 26.55 | 100 | 24 | |
| 7. | Shashur | 21.10 × 19.2616.13 × 16.13 | 86.0313.97 | 70 | |
The diploid(n=8) cytotype
Only one population growing on the moist alpine slopes of Gauri Kund (3930 m) in the Manimahesh hills (Chamba district) existed at diploid level (based on x=8) as confirmed from the presence of 8 medium sized bivalents in the PMCs at MI (Fig. 3, A). These bivalents showed regular segregation during AI. Further meiotic course was also regular resulting into normal tetrad formation, nearly cent per cent pollen fertility and uniform sized pollen grains.
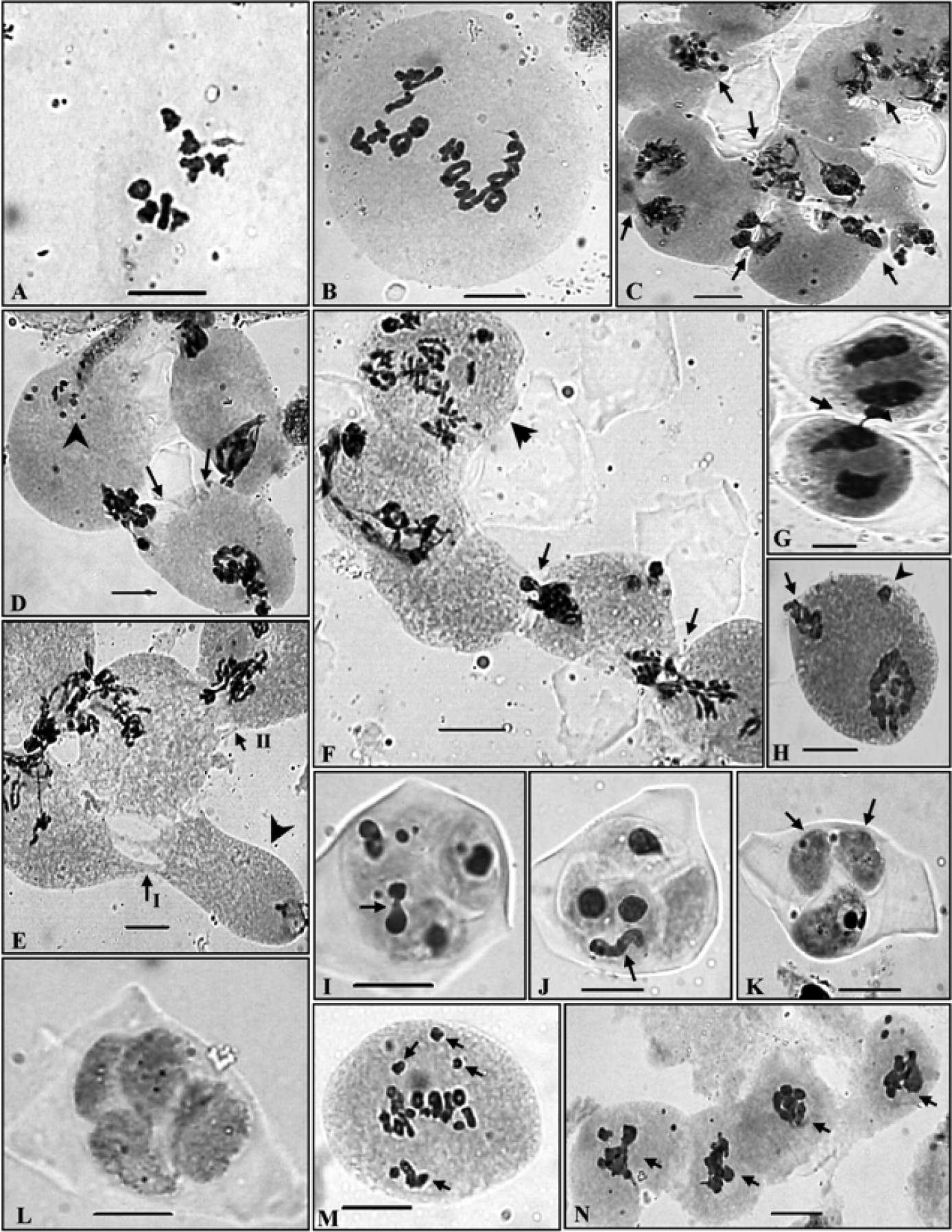
PMCs showing meiotic chromosome number and abnormal meiotic behaviour in Ranunculus hirtellus. A 2× cytotype, n=8 at MI B 4× cytotype, n=16 at diakinesis C A group of PMCs involved in the transfer of chromatin material at early prophase-I (arrowed) D Two PMCs (arrowed) showing simultaneous transfer of chromatin material and pycnotic chromatin material (arrowhead) E A group of PMCs showing narrow and broad cytoplasmic connections (arrowed) and an almost enucleated PMC (arrowhead) F A group of PMCs showing transfer of chromatin material (arrowed) and a hyperploid PMC (arrowhead) G Two PMCs involved in chromatin material transfer at TI (arrowed) H A PMC at TI showing broken chromatin strand at one pole (arrowed) and a laggard (arrowhead) I, J Microspores showing transfer of chromatin material within the sporads (arrowed) K Two empty microspores (arrowed) without any chromatin material in a sporad L Completely empty microspores in a sporad M Out of metaphase plate bivalents (arrowed) N A group of PMCs showing chromatin stickiness (arrowed).
The tetraploid (n=16) cytotype
The tetraploid cytotype has been found to be more common as confirmed from the presence of meiotic chromosome number of n=16 in six out of the seven populations scored presently from the different localities in the Himalayas. These tetraploid individuals in all the populations unequivocally showed the presence of 16 bivalents in the PMCs (Fig. 3, B). In spite of normal bivalent/s formation and their equal distribution during anaphases, PMCs showed various meiotic abnormalities which include PMCs involved in chromatin transfer at different stages (Fig. 3, C-L), out of plate bivalent/s (Fig. 3, M), chromatin stickiness (Fig. 3, N), pycnotic chromatin (Fig. 3, D), extra chromatin in PMCs (Fig. 4, A), supernumerary nucleoli (Fig. 4, B, I), laggards and chromatin bridges (Fig. 4, C-E), micronuclei (Fig. 4, I, J) and disoriented chromatin material at anaphases/telophases (Fig. 4, F-H). Consequent to these meiotic abnormalities, abnormal sporads (Fig. 4, J-L) were produced which lead to varying percentages of pollen sterility (24 - 77 %) and pollen grains of heterogeneous sizes (Fig. 4, M & N). The data on cytomixis, meiotic course, microsporogenesis and pollen sterility and pollen size in each population of the tetraploid cytotype are provided in the Tables 3, 4.
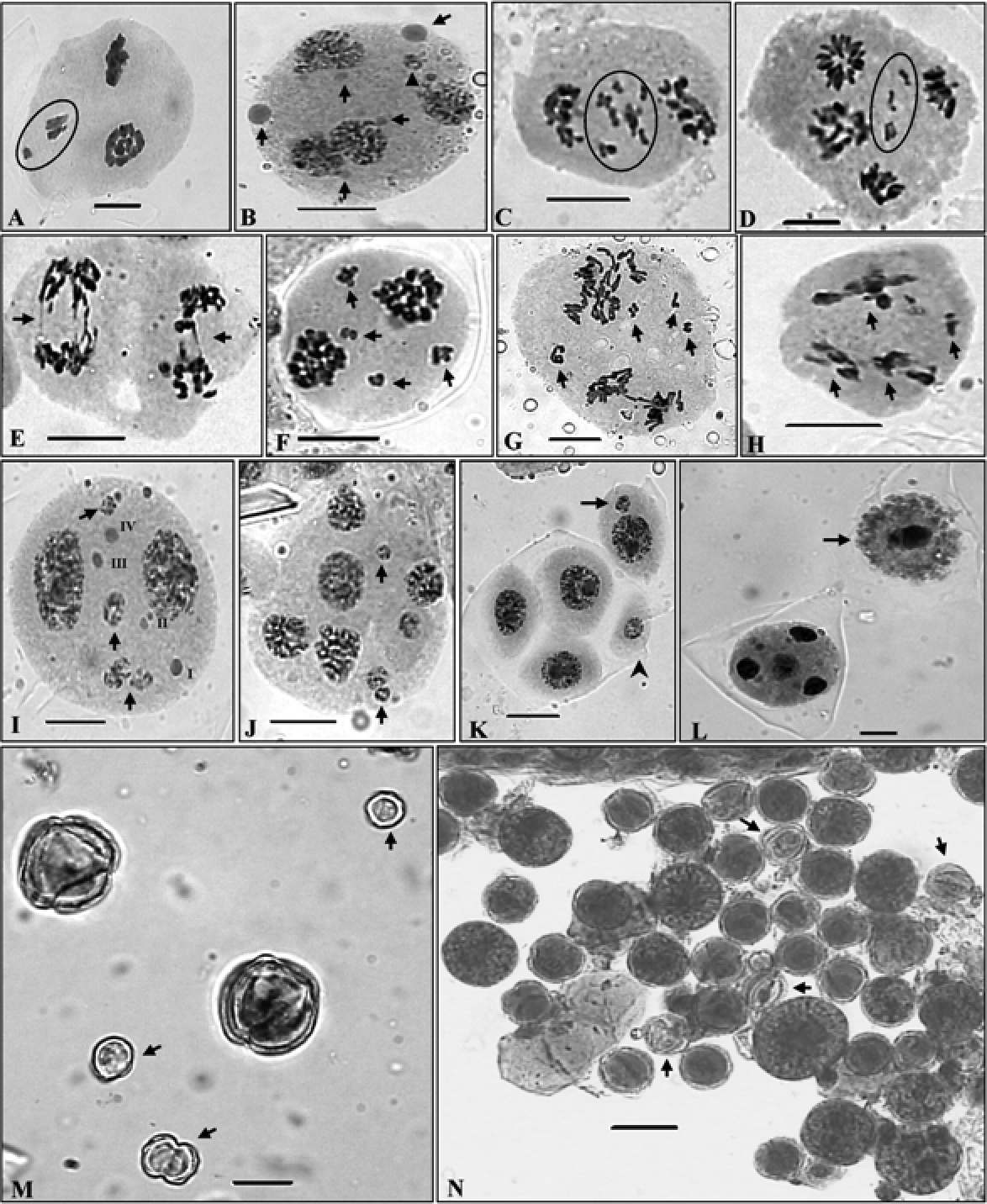
Meiotic abnormalities and pollen grains. A A PMC showing extra chromatin material (encircled) B A PMC showing unequal sized supernumerary nucleoli (arrowed) and micronuclei (arrowhead) C, D Laggards at late AI/II (encircled) E Chromatin bridges at AII (arrowed) F–H PMCs showing disoriented chromosomes in multiple groups (arrowed) I A PMC with micronuclei (arrowed) and supernumerary nucleoli (I–IV) J A polyad with micronuclei (arrowed) K Sporad with included micronuclei in microspore (arrowed) and a microcyte (arrowhead) L A monad (arrowed) and a normal PMC with four haploid nuclei at TII M Very small sized sterile/unstained (arrowed) and large lightly stained pollen grains N Stained apparently fertile heterogeneous sized and sterile/unstained (arrowed) pollen grains. Scale bars = 10 μm, except micrograph N=20 μm
Cytomixis, meiotic course and microsporogenesis in the 4× cytotype of Ranunculus hirtellus. PMC = pollen mother cell; M-I = metaphase-I; P-I = Prophase-I; AI/TI= anaphase-I/telophase-I; AII/TII = anaphase-II/telophase-II;
| Populations | Cytomixis | Meiotic course | Microsporogenesis | |||||
|---|---|---|---|---|---|---|---|---|
| % age of PMCs involved | No. of PMCs involved | Meiotic stage/s | PMCs with chromosome stickiness (%) | PMCs with laggards at AI/TI, AII/TII (%) | PMCs with bridges at AI/TI, AII /TII (%) | PMCs with unoriented chromatin material (%) | Abnormal sporads (tetrads and polyads with and without micronuclei) | |
| Dhancho | 5.33 | 2-3 | M-I | 18.10 | 35.90 | 1.93 | --- | --- |
| Manimahesh Lake | 15.95 | 2-3 | P-I | --- | 6.83 | --- | --- | --- |
| Jalori Pass | 26.40 | 2-5 | P-I, M-I | --- | 11.40 | 2.50 | --- | --- |
| Rohtang Pass | 22.85 | 2-4 | M-I | --- | 53.85 | --- | 30.80 | --- |
| Keylong | 26.47 | 2-3 | M-I | --- | 5.03 | --- | --- | 15.55 |
| Shashur | 26.17 | 2-4 | P-I, M-I, T-I | --- | 26.53 | --- | --- | 44.49 |
Cytomixis involving inter PMC transfer of chromatin material was observed only during the meiotic stages of meiosis-I (Table 4). The chromatin transfer which occurred through narrow as well as broad cytomictic channels among 2–5 proximate PMCs leads to the formation of PMCs with extra chromatin material (Fig. 3, C-H & 4, A). Transfer of chromatin material among PMCs was observed to be both unidirectional as well as bidirectional forming 1–2 chromatin strands. Hypo-, hyperploid and enucleated PMCs were resulted due to partial and complete transfer of chromatin material (Fig. 3, E, F). Interestingly in few instances transfer of chromatin material occurred simultaneously from two PMCs to a single recipient PMC (Fig. 3, D). In some cases remnants of chromatin strands which existed between proximate PMCs during cytomixis were seen as broken chromatin strands (Fig. 3, H). Out of plate bivalent/s at MI was also noticed in a few PMCs (Fig. 3, M). Chromatin stickiness mostly existed in the meiocytes at MI (Fig. 3, N, Table 4). Pycnotic chromatin formed due to chromatin stickiness was also noticed at earlier stages of prophase-I (Fig. 3, D). Some PMCs also showed the presence of supernumerary nucleoli which were of unequal sizes (Fig. 4, B, I). Other most prominent meiotic anomalies noticed were the occurrence of laggards (1–7) at anaphases/ telophases (Fig. 4, C & D, Table 3) and disorientation of chromosomes during anaphases owing to spindle irregularities (Fig. 4, F-H, Table 4). These laggards and unoriented chromatin material failed to get included at poles during telophases, and constituted micronuclei, multipolar PMCs and microcytes (small sized microspore) during sporad formation (Fig. 4, K). The number of such micronuclei in PMCs varied from 1–4 (Fig. 4, I & J). During microsporogenesis these micronuclei were observed to present either freely in the sporads along with four microspores (1–3 micronuclei as separate units) or as included in microspores (Fig. 4, K). Polyads with 1–2 micronuclei and without micronuclei were also noticed. Chromatin bridges were also observed during late AII/TII stages Fig. 4, E, Table 4). Another interesting observation in the population collected from Dhancho (3, 030 m)was the occurrence of sporads with empty microspores i.e. microspores without any chromatin material in 6.40 % of cases (Fig. 3, K). Sporads with all the microspores without any chromatin material were also observed (Fig. 3, L). Transfer of chromatin within the sporad units has also been observed in some cases (Fig. 3, I & J). Besides, monads were also recorded in 2.4 % of the observed cases in the same population (Fig. 4, L). Chromatin transfer coupled with associated meiotic abnormalities and consequent abnormal microsporogenesis resulted into high pollen sterility (Table 3) and heterogeneous sized pollen grains (Fig. 4, L, Table 4).
Discussion Chromosomal status, comparison of 2× and 4× cytotypes and their distributional pattern in Indian HimalayasThe present diploid (n=8) and tetraploid (n=16)
chromosome counts for the species from this region of the Himalayas,
explored for the first time, agree with the earlier reports from other
regions of Indian Himalayas. Both the diploid, 2n=2×=16 (
The two intraspecific cytotypes (2×, 4× at x=8) here recorded in Ranunculus hirtellus
from the Northwest Himalayas showed variation in vegetative and
reproductive characters. Analysis of various macro- and microscopic
characters in individuals with 2x and 4x cytotypes revealed that
increase in ploidy level is correlated with gigantism for vegetative
(plant height, radical leaf length), stomatal (density, size and
index) characters and number of flowers/plant. Consequently, the
individuals of 2× and 4× cytotypes of Ranunculus hirtellus
can be distinguished from each other in the field. The 4× plants were
much taller in size, and had large leaves. It is thus apparent that
morphological characters in the intraspecific 2× and 4× cytotypes of Ranunculus hirtellus are directly correlated with the increase in ploidy level as had been reported earlier in Capsella bursa-pastoris (L.) Medik., 1792(
The male meiotic course in the meiocytes was perfectly normal in the diploid cytotype resulting into cent percent pollen fertility. However, all the individuals of the 4× cytotype showed the phenomenon of cytomixis involving chromatin transfer among proximate PMCs and various other associated meiotic abnormalities. Consequently very high pollen sterility and fertile pollen grains of two heterogeneous sizes were resulted. The phenomenon of cytomixis is reported here for the first time in the species.
Cytomixis in the PMCs of tetraploid cytotypeTransfer of chromatin material between the adjacent
PMCs occurred through cytomictic channels and these cytoplasmic channels
originating from the pre-existing connections of plasmodesmata formed
within the anther tissues. As meiosis progress these connections get
obstructed by the callose plugs. However, in some cases they may exist
till the later stages of meiosis and their size may increase to form
conspicuous inter-PMC cytomictic channels through which transfer of
chromatin or chromosomes may take place (
Chromatin transfer occurred through variable sized
cytoplasmic channels forming 1–2 chromatin strands involving 2–5 PMCs
and the percentage of meiocytes involved in cytomixis ranged between
5.53–26.47%. The chromatin material transfer was observed only during
the early stages of the meiosis-I, which confirmed the view of other
workers that earlier stages of meiosis-I are more favourable for
cytomixis (
Another rare and interesting observation recorded during the meiotic course of Ranunculus hirtellus was the occurrence of sporads with empty microspores. In some cases sporads were devoid of any chromatin material. One of the possible explanations for the presence of empty microspores in a sporad is the transfer of chromatin within the sporad. Completely empty sporads might have resulted due to the transfer of chromatin between the units of two different sporads. To the best of our knowledge this is the first report of the occurrence of empty microspore units in sporads which were devoid of chromatin material due to complete transfer of chromatin material among microspores of sporads.
Other meiotic abnormalitiesThe other most frequently observed meiotic
abnormalities included laggards and bridges at anaphase/telophase,
chromatin stickiness, pycnotic chromatin and aberrant spindle activity
in the PMCs which possibly have been induced by cytomixis (
The phenomenon of cytomixis has been reported a large
number of angiospermic plants, and many workers consider cytomixis to
be of considerable evolutionary significance (
On the basis ofmorphological characters both the 2× and 4× cytotypes are distinguishable in the field from each other. The 4× cytotype has a wider distribution in the Indian Himalayas compared to 2× and 3× cytotypes. And the occurrence of various meiotic abnormalities in the 4× cytotype may be attributed to the genetic imbalance in the 4× cytotype, high altitude and low temperature stress conditions prevailing in the cold deserts.
The authors are grateful to the University Grants Commission, New Delhi for providing financial assistance under the DRS SAP I, II and III and ASIST programme and CSIR for providing Senior Research Fellowship to senior author. Further support was provided by the UGC under the Dr. D.S. Kothari Post- Doctoral Fellowship Scheme {Award Letter No. F.4-2/2006 (BSR)/13-427/2011(BSR)} to Dr. Puneet Kumar. Thanks are also due to the Head, Department of Botany for necessary laboratory and library facilities. The authors are very grateful to the editor and anonymous reviewers for their assistance and valuable suggestions, which were helpful in improving this paper.