(C) 2011 Tatiane Ramos Sampaio. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In the present work, six curimatid species were analyzed: Cyphocharax voga (Hensel, 1870), Cyphocharax spilotus (Vari, 1987), Cyphocharax saladensis (Meinken, 1933), Cyphocharax modestus (Fernández-Yépez, 1948), Steindachnerina biornata (Braga & Azpelicueta, 1987) and Steindachnerina insculpta (Fernández-Yépez, 1948) collected from two hydrographic basins. All samples presented 2n=54 meta-submetacentric (m-sm) chromosomes and FN equal to 108, and 1 or 2 B microchromosomes in the mitotic and meiotic cells of the six sampled populations showing inter-and intraindividual variation. The analysis of the meiotic cells in Cyphocharax saladensis, Cyphocharax spilotus, and Cyphocharax voga showed a modal number of 54 chromosomes in the spermatogonial metaphases and 27 bivalents in the pachytene, diplotene, diakinesis and in metaphase I stages, and 27 chromosomes in metaphase II; in Cyphocharax modestus, Steindachnerina biornata, and Steindachnerina insculpta, spermatogonial metaphases with 54 chromosomes and pachytene and metaphase I with 27 bivalents were observed. The B microchromosome was observed as univalent in the spermatogonial metaphase of Cyphocharax spilotus, in the pachytene stage in the other species, with the exception of Cyphocharax saladensis, and Steindachnerina biornata in metaphase I. New occurrences of the B microchromosome in Cyphocharax voga, Cyphocharax saladensis and Steindachnerina biornata were observed, confirming that the presence of this type of chromosome is a striking characteristic of this group of fish.
B microchromosome, meiosis, curimatids
B chromosomes, also known as supernumerary or accessory chromosomes, are additional dispensable chromosomes present in some individuals of some populations in some species. They have probably originated from the A complement, but followed their own evolutionary paths, being found in different groups of both animals and plants (
The irregular behavior of this chromosome type in mitosis and in meiosis causes it to accumulate selfishly in the germ line of many species, producing a non-Mendelian segregation with transmission rates higher than those yielded by the chromosomes of the A complement (
In freshwater Neotropical fish, the occurrence of B chromosomes has been reported in 61 species, distributed in 16 families of seven different orders and in distinct hydrographic basins, according with the revision accomplished by
The first work to record the presence of the B chromosome in the family Curimatidae was carried out by
The current study examines the frequency, behavior and distribution of B microchromosomes in mitotic and meiotic cells in six fish species of the family Curimatidae from two hydrographic basins.
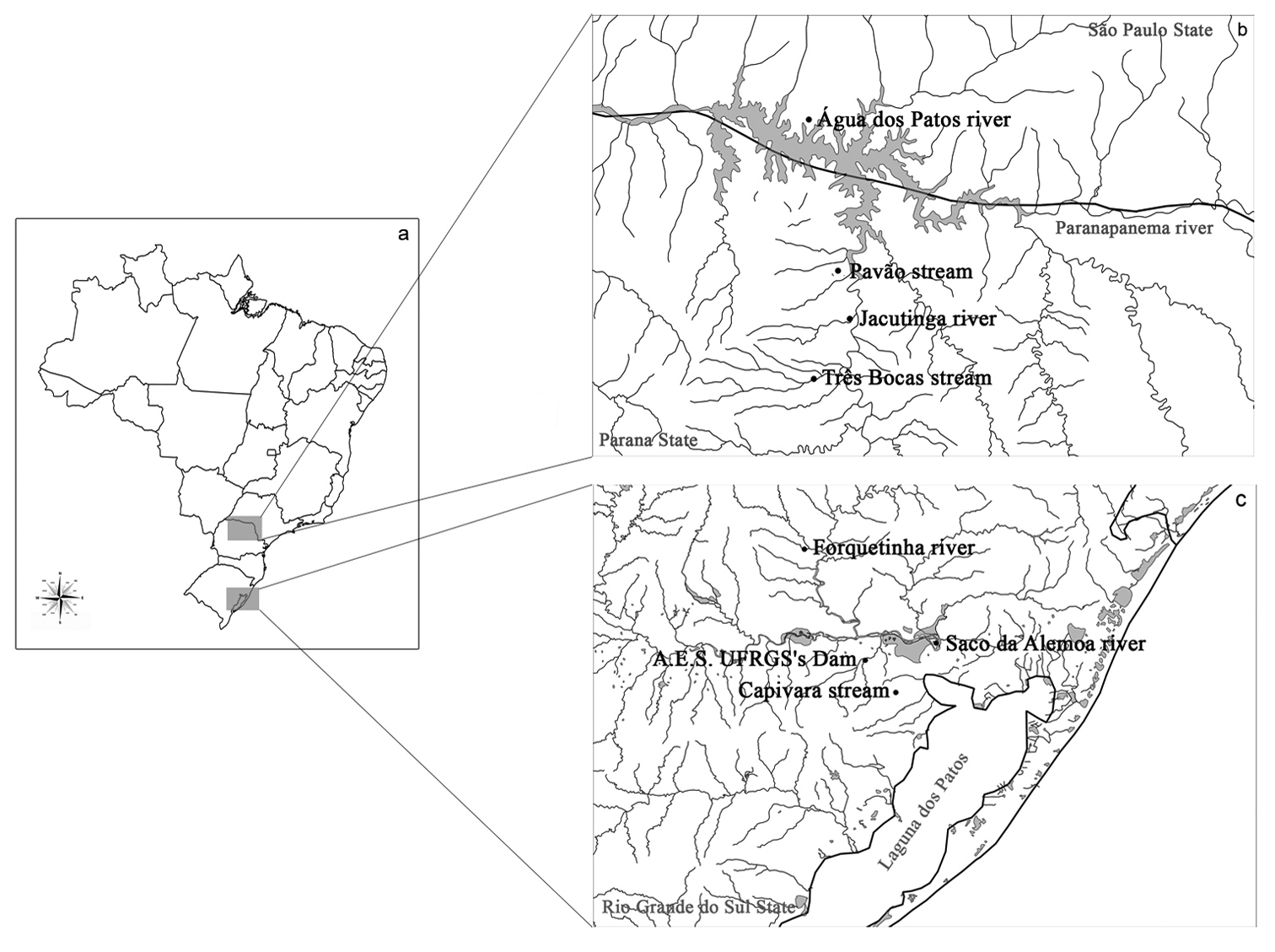
Material and methodsSix species of the family Curimatidae were analysed: Cyphocharax voga (Hensel, 1870), Cyphocharax spilotus (Vari, 1987), Cyphocharax saladensis (Meinken, 1933), Cyphocharax modestus (Fernández-Yépez, 1948), Steindachnerina biornata (Braga & Azpelicueta, 1987)and Steindachnerina insculpta (Fernández-Yépez, 1948), collected from the Laguna dos Patos Hydrographic System/RS and Paranapanema River basin/SP/PR (Fig. 1, Table 1). Voucher specimens are catalogued in the Zoology Museum of the Universidade Estadual de Londrina, Paraná state, under catalog numbers: MZUEL 5105 – Cyphocharax voga; MZUEL 5106 – Cyphocharax spilotus; MZUEL 5058 – Cyphocharax saladensis; MZUEL 1374 – Cyphocharax modestus; MZUEL 5059 – Steindachnerina biornata and MZUEL 1042 – Steindachnerina insculpta.
a Map of Brazil b Collection sites of Paranapanema River basin: Água dos Patos River in the São Paulo state, Pavão stream, Jacutinga River and Tres Bocas stream in the Parana state c Collection sites of Laguna dos Patos Hydrographic System: Forquetinha River, Saco da Alemoa River, Agronomic Experiment Station of UFRGS’s Dam and Capivara stream in the Rio Grande do Sul state.
Species analysed, collection sites and hydrographic basins.
| Species | Number of individuals | Collection sites | Basins |
| Cyphocharax voga | 1♀, 1♂ | Saco da Alemoa River, Eldorado do Sul, RS, Brazil S29°59'15.6", W51°14'24.1" |
Laguna dos Patos Hydrographic System |
| 2♀, 9♂ | Capivara stream, Barra do Ribeiro, RS, Brazil S30°17'33.3", W51°19'23.6" |
||
| Cyphocharax spilotus | 2♀, 3♂ | Capivara stream, Barra do Ribeiro, RS, Brazil S30°17'33.3", W51°19'23.6" |
|
| Cyphocharax saladensis | 1♀, 10♂ | Agronomic Experiment Station of UFRGS’s Dam, Eldorado do Sul, RS, Brazil S30°05'36.2", W51°40'41.8" |
|
| Steindachnerina biornata | 1♀, 1♂ | Forquetinha River, Canudos do Vale, RS, Brazil S29°19'20.9", W50°14'3.6" |
|
| Cyphocharax modestus | 2♀, 5♂ | Tres Bocas stream, Londrina, PR, Brazil S23°17'12.9", W51°13'58.2" |
Paranapanema River |
| Steindachnerina insculpta | 3♂ | Tres Bocas stream, Londrina, PR, Brazil S23°17'12.9", W51°13'58.2" |
|
| 2♂ | Pavão stream, Sertanópolis, PR, Brazil | ||
| 4♀, 8♂ | Jacutinga River, Londrina, PR, Brazil S23°23'6.6", W51°04'35.8" |
||
| 1♀, 5♂ | Água dos Patos River, Iepê, SP, Brazil S22°41'17.7", W51°05'23.9" |
Mitotic chromosomes were obtained by direct preparation removing the anterior kidney, according to
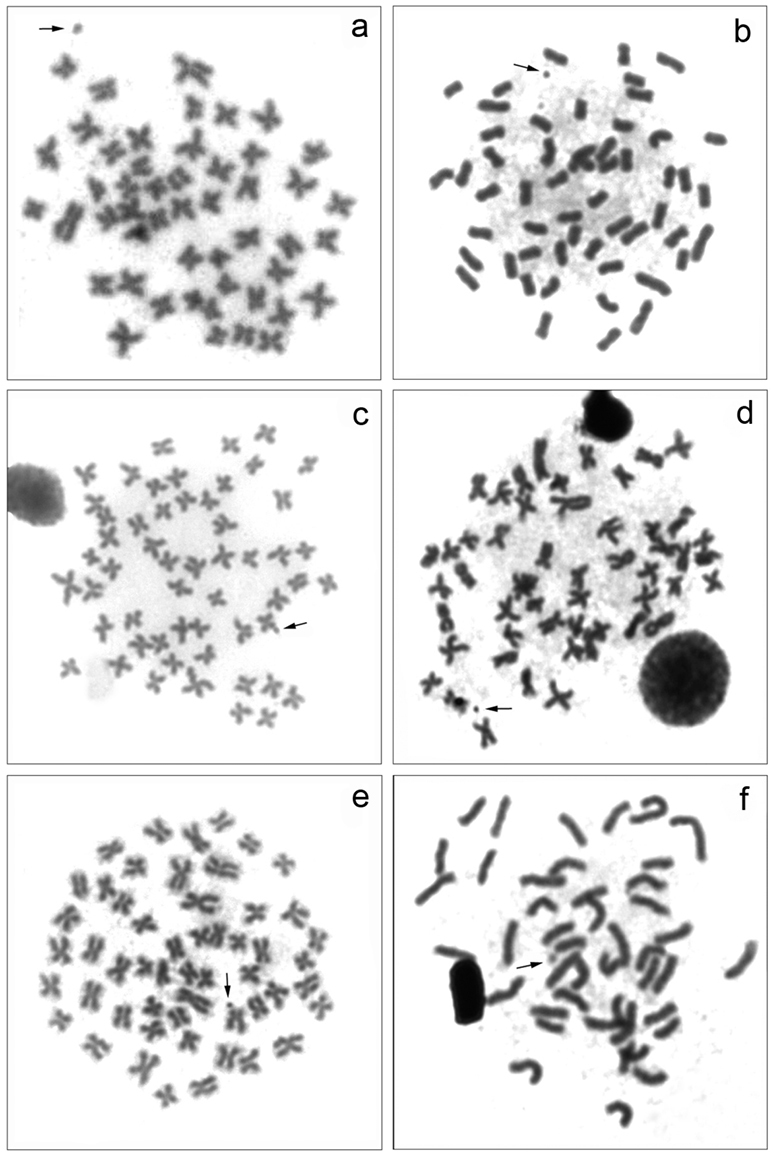
All samples analyzed showed a diploid number of 54 meta-submetacentric chromosomes (m-sm) and a fundamental number (FN) equal to 108 (Fig. 2). This karyotype structure is often found in this fish group, and are conservative among the species of the family Curimatidae, as already observed by
Somatic metaphases: a Cyphocharax voga b Cyphocharax spilotus c Cyphocharax saladensis d Cyphocharax modestus e Steindachnerina biornata f Steindachnerina insculpta. The arrows indicate the B microchromosome.
One B microchromosome was observed in all populations studied, with variation in the number and frequency among them (Fig. 2). In the species Cyphocharax voga, Cyphocharax spilotus, Cyphocharax saladensis and Steindachnerina biornata belonging to the Laguna dos Patos Hydrographic System, there was an inter-and intraindividual variation from 0 to 1 B microchromosome in the somatic cells (Table 2). In Cyphocharax modestus and Steindachnerina insculpta, from the Paranapanema River basin, up to two B microchromosomes, also exhibiting inter-and intraindividual variation, were detected in the somatic cells (Table 3). As proposed by
B microchromosome frequency in somatic cells of the curimatids from Laguna dos Patos Hydrographic System/RS.
| Species | Locality | Specimens | Sex | Number of B chromosome | Total number of cells | |
| 0 | 1 | |||||
| Cyphocharax voga | Saco da Alemoa River | 149 | ♀ | 22 | 2 | 24 |
| 150 | ♂ | 3 | 0 | 3 | ||
|
Total % |
25 92, 6 |
2 7, 4 |
27 | |||
| Capivara stream | 748 | ♀ | 3 | 1 | 4 | |
| 752 | ♂ | 4 | 1 | 5w | ||
| 755 | ♀ | 17 | 0 | 17 | ||
| 777 | ♂ | 42 | 1 | 43 | ||
| 780 | ♂ | 12 | 0 | 12 | ||
|
Total % |
78 96, 3 |
3 3, 7 |
81 | |||
| Cyphocharax spilotus | Capivara stream | 580 | ♂ | 2 | 0 | 2 |
| 753 | ♀ | 25 | 3 | 28 | ||
| 758 | ♀ | 23 | 0 | 23 | ||
| 778 | ♂ | 8 | 1 | 9 | ||
| 779 | ♂ | 22 | 0 | 22 | ||
|
Total % |
80 95, 2 |
4 4, 8 |
84 | |||
| Cyphocharax saladensis | Agronomic Experiment Station of UFRGS’s Dam | 784 | ♂ | 4 | 0 | 4 |
| 786 | ♀ | 5 | 0 | 5 | ||
| 787 | ♂ | 6 | 0 | 6 | ||
| 788 | ♂ | 36 | 1 | 37 | ||
| 789 | ♂ | 10 | 0 | 10 | ||
| 790 | ♂ | 8 | 0 | 8 | ||
| 791 | ♂ | 7 | 2 | 9 | ||
| 792 | ♂ | 3 | 0 | 3 | ||
| 793 | ♂ | 2 | 0 | 2 | ||
| 794 | ♂ | 6 | 0 | 6 | ||
|
Total % |
87 96, 7 |
3 3, 3 |
90 | |||
| Steindachnerina biornata | Forquetinha River | 857 | ♀ | 55 | 3 | 58 |
| 996 | ♂ | 3 | 2 | 5 | ||
|
Total % |
58 92 |
5 8 |
63 | |||
B microchromosome frequency in somatic cells of the curimatids from Paranapanema River basin.
| Species | Locality | Specimens | Sex | Number of B chromosome | Total number of cells | ||
| 0 | 1 | 2 | |||||
| Cyphocharax modestus | Tres Bocas stream | 2656 | ♂ | 5 | 0 | 0 | 5 |
| 3815 | ♂ | 18 | 0 | 0 | 18 | ||
| 3909 | ♀ | 8 | 0 | 0 | 8 | ||
| 3992 | ♀ | 46 | 3 | 1 | 50 | ||
|
Total % |
77 95 |
3 3, 75 |
1 1, 25 |
81 | |||
| Steindachnerina insculpta | Pavão stream | 3277 | ♂ | 3 | 2 | 0 | 5 |
| 3278 | ♂ | 8 | 0 | 0 | 8 | ||
|
Total % |
11 84, 6 |
2 15, 4 |
0 0 |
13 | |||
| Água dos Patos River | 3393 | ♀ | 40 | 8 | 0 | 48 | |
| 3407 | ♂ | 18 | 0 | 0 | 18 | ||
| 3408 | ♂ | 11 | 2 | 1 | 14 | ||
| 3409 | ♂ | 21 | 0 | 0 | 21 | ||
| 3411 | ♂ | 22 | 0 | 0 | 22 | ||
| 3745 | ♂ | 5 | 0 | 0 | 5 | ||
|
Total % |
117 91, 4 |
10 7, 8 |
1 0, 8 |
128 | |||
| Jacutinga River | 3453 | ♀ | 15 | 2 | 1 | 18 | |
| 3454 | ♀ | 22 | 0 | 0 | 22 | ||
| 3461 | ♂ | 14 | 0 | 0 | 14 | ||
| 3462 | ♂ | 20 | 0 | 0 | 20 | ||
| 3465 | ♂ | 23 | 1 | 0 | 24 | ||
| 3862 | ♀ | 6 | 0 | 0 | 6 | ||
| 3986 | ♂ | 2 | 0 | 0 | 2 | ||
| 3987 | ♂ | 5 | 0 | 0 | 5 | ||
| 3991 | ♂ | 4 | 0 | 0 | 4 | ||
| 3993 | ♀ | 3 | 0 | 0 | 3 | ||
| 4046 | ♂ | 8 | 0 | 0 | 8 | ||
| 4049 | ♂ | 4 | 10 | 0 | 14 | ||
|
Total % |
126 90 |
13 9, 3 |
1 0, 7 |
140 | |||
Of the total number of somatic cells with B microchromosomes analyzed in six species of Curimatids, there was a variation from 3.3% in Cyphocharax saladensis to 15.4% in Steindachnerina insculpta. Among the species belonging to the Laguna dos Patos Hydrographic System, Cyphocharax voga showed the highest percentage of B cells (11.1%), followed by Steindachnerina biornata with 8%, Cyphocharax spilotus with 4.8%, and Cyphocharax saladensis with 3.3% (Table 2).
In the Paranapanema River basin, the population of the Steindachnerina insculpta from the Pavão stream/PR showed 15.4% of their somatic cells with B microchromosomes, followed by the populations of the Jacutinga River/PR with 10% and Água dos Patos River/SP with 8.6%. The species Cyphocharax modestus from the Tres Bocas stream/PR presented 5% of their cells with B microchromosomes (Table 3). The data collected from both basins corroborate the constant presence of this type of chromosome in the Curimatidae family, constituting a striking characteristic of the group, even when its incidence is low.
Specimens of Cyphocharax voga collected at two localities in the Laguna dos Patos Hydrographic System (Saco da Alemoa River and Capivara stream) not presented interpopulation differences in the number and frequency of the Bs. Likewise were not observed significant differences between the four populations of Steindachnerina insculpta, belonging to Paranapanema River basin.
The B microchromosome was observed in four species of curimatids collected from different populations: Cyphocharax gouldingi (
Cytogenetic date of differents species of curimatids (2n: diploid number; FN: number fundamental; Bs: supernumerary chromosomes).
| Species | 2n | FN | Bs | B Size | References* |
| Curimata cyprinoides | 54 | 108 | - | - | 3, 15 |
| Curimata inornata | 54 | 108 | - | - | 3, 15 |
| Curimata kneri | 54 | 108 | - | - | 3 |
| Curimata ocellata | 56 | 112 | - | - | 3 |
| Curimata vittata | 54 | 108 | - | - | 3 |
| Curimatella alburna | 54 | 108 | - | - | 3 |
| Curimatella dorsalis | 54 | 108 | - | - | 8, 12 |
| Curimatella imaculata | 54 | 108 | - | - | 15 |
| Curimatella lepidura | 54 | 108 | - | - | 2 |
| Curimatella meyeri | 54 | 108 | - | - | 3 |
| Curimatopsis myersi | 46 | 46 | - | - | 8 |
| Cyphocharax gilbert | 54 | 108 | - | - | 6, 15 |
| Cyphocharax cf. gillii | 54 | 108 | - | - | 2 |
| Cyphocharax gouldingi | 54 | 108 | 0 - 1 | Micro | 15 |
| Cyphocharax modestus | 54 | 108 | 0 - 4 | Micro | 1, 2, 7, 9, 13, 14, 16, 17, 18 |
| Cyphocharax nagelii | 54 | 108 | - | - | 2, 15 |
| Cyphocharax cf. spilurus | 54 | 108 | - | - | 2 |
| Cyphocharax spilotus | 54 | 108 | 0 - 1 | Micro | 11, 12, 18 |
| Cyphocharax vanderi | 54 | 108 | - | - | 2 |
| Cyphocharax voga | 54 | 108 | 0 - 1 | Micro | 2, 12, 18 |
| Cyphocharax platanus | 58 | 116 | - | - | 12, 15 |
| Cyphocharax saladensis | 54 | 108 | 0 - 1 | Micro | 18 |
| Potamorhina altamazonica | 102 | 106 | - | - | 4 |
| Potamorhina latior | 56 | 112 | - | - | 4 |
| Potamorhina pristigaster | 54 | 108 | - | - | 4 |
| Potamorhina squamoralevis | 102 | 106 | - | - | 12 |
| Psectrogaster amazonica | 54 | 108 | - | - | 15 |
| Psectrogaster curviventris | 54 | 108 | - | - | 8, 12 |
| Psectrogaster rutiloides | 54 | 108 | - | - | 3 |
| Steindachnerina amazonica | 54 | 108 | - | - | 15 |
| Steindachnerina biornata | 54 | 108 | 0 - 1 | Micro | 18 |
| Steindachnerina brevipina | 54 | 108 | - | - | 8, 12 |
| Steindachnerina conspersa | 54 | 108 | - | - | 2, 12 |
| Steindachnerina elegans | 54 | 108 | - | - | 2 |
| Steindachnerina gracilis | 54 | 108 | - | - | 15 |
| Steindachnerina cf. guentheri | 54 | 108 | - | - | 10 |
| Steindachnerina insculpta | 54 | 108 | 0 - 2 | Micro | 2, 5, 13, 14, 15, 17, 18 |
| Steindachnerina leucisca | 54 | 108 | - | - | 3 |
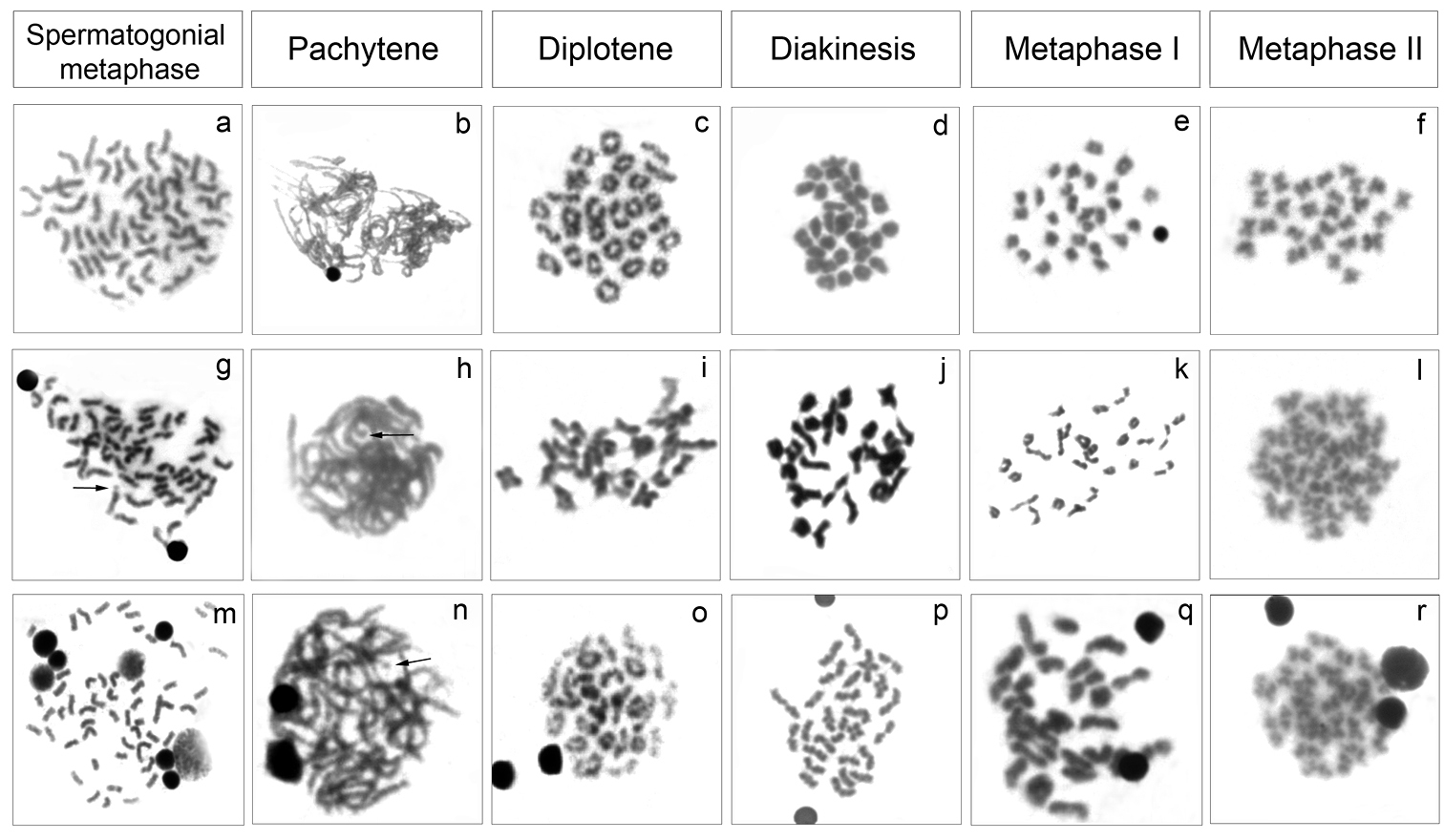
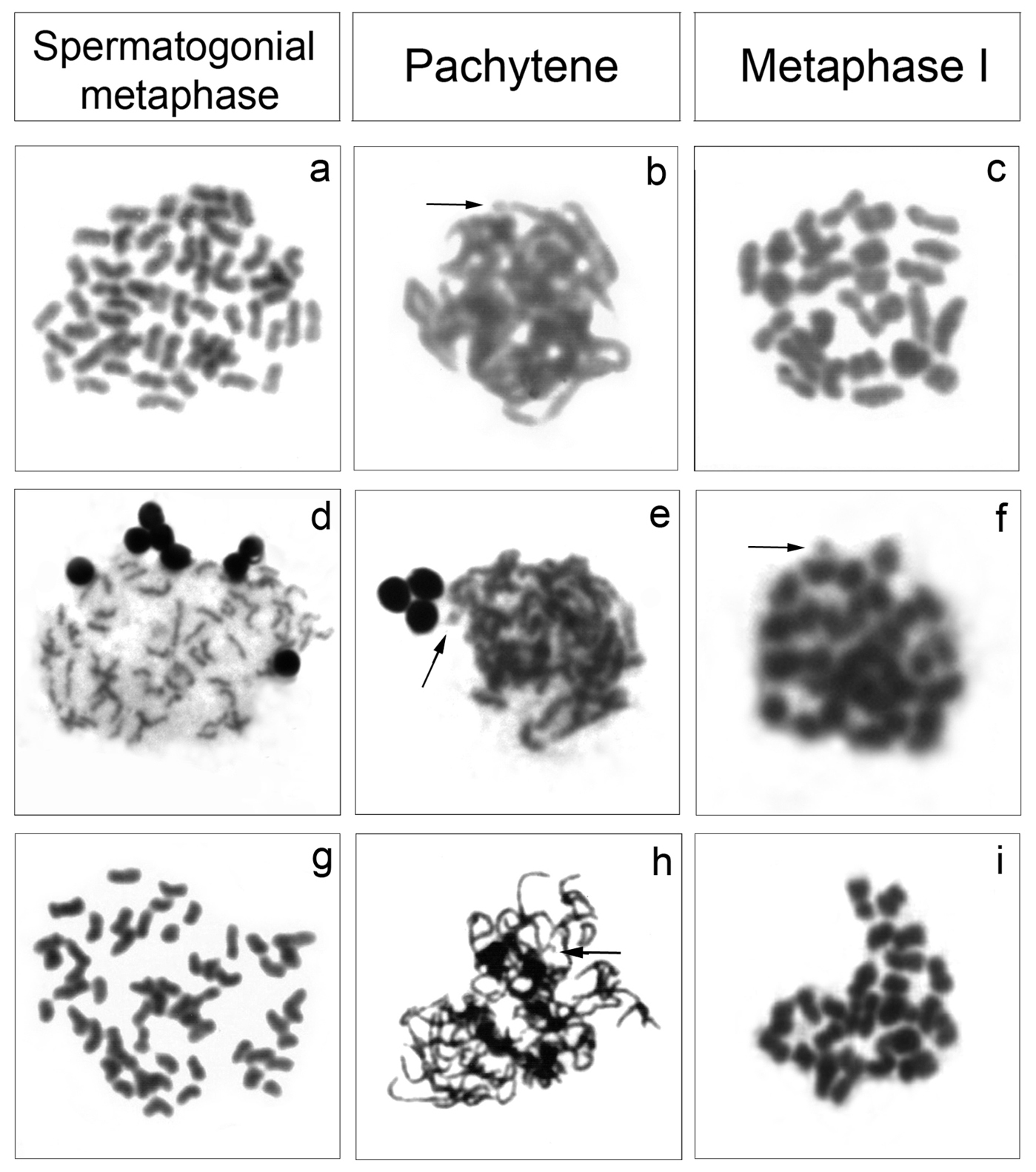
The analysis of meiotic cells in Cyphocharax saladensis, Cyphocharax spilotus and Cyphocharax voga showed a modal number of 54 chromosomes in spermatogonial metaphases and 27 bivalents in the stages of pachytene, diplotene, diakinesis and metaphase I, and 27 chromosomes in metaphase II (Fig. 3). In Cyphocharax modestus, Steindachnerina biornata and Steindachnerina insculpta, spermatogonial metaphases with 54 chromosomes and pachytene and metaphase I with 27 bivalents were observed (Fig. 4). It was possible to observe the B microchromosome as univalent in the spermatogonial metaphase in Cyphocharax spilotus; in the pachytene stage in Cyphocharax spilotus, Cyphocharax voga, Cyphocharax modestus, Steindachnerina biornata and Steindachnerina insculpta; and in metaphase I in Steindachnerina biornata (Figs 3, 4).
Meiotic cells: Cyphocharax saladensis a–f Cyphocharax spilotus g–l and Cyphocharax voga m–r belonging to Laguna dos Patos Hydrographic System. The arrows indicate the B microchromosome univalent in g, h and n.
Meiotic cells: Cyphocharax modestus a–c, Steindachnerina biornata d–f and Steindachnerina insculpta g–i. The arrows indicate the B microchromosome univalent in b, e, f and h.
In both types of cell division, the number of cells without B microchromosomes was greater than number of cells with B microchromosomes in the species of Curimatidae.
In others groups of fishes with B-chromosomes meiotic analysis has been performed in order to understand the behavior of this chromosome, as in Prochilodus lineatus (Valenciennes, 1836) from the Mogi Guaçu River (Pirassununga/SP), whose studies of the synaptonemal complex showed that no B chromosome paired with autosomal chromosomes. In the late pachytene stage, 27 paired bivalents and small bivalent, trivalent and quadrivalent B chromosomes were observed. The pairing of B chromosomes was interpreted as a result of homology between these chromosomes (
The meiotic data presented in this study are the first for Curimatidae, and also indicate the instability of the B microchromosome during meiosis, demonstrating that this chromosome has no homology with any normal chromosome complement in these species. Analyses of the synaptonemal complex in the analyzed species would be interesting to complement the study of the meiotic behavior of B microchromosome in the species Curimatidae.
According
There are two hypotheses that could explain the origin of B chromosomes in Cyphocharax modestus and Steindachnerina insculpta, according
The results obtained in this study provides more information about the occurrence of B microchromosomes in the curimatids, confirming its presence in Cyphocharax spilotus, Cyphocharax modestus and Steindachnerina insculpta, previously described in other populations, and showing new events in Cyphocharax voga, Cyphocharax saladensis and Steindachnerina biornata. These dataconfirm the outstanding characteristic of this type of chromosome in this group of fish and its mitotic and meiotic instability and allow a further discussion about the origin of Bs in the family Curimatidae.
The authors are grateful to CNPq for fellowships, to Dr. Luiz R. Malabarba for the identification of the studied species from the Laguna dos Patos Hydrographic System/RS and MSc Larissa Bettin Pires and Fábio H. Takagui for assistance in the construction of maps.