(C) 2012 Lucas C. Majure. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Ploidy has been well studied and used extensively in the genus Opuntia to determine species boundaries, detect evidence of hybridization, and infer evolutionary patterns. We carried out chromosome counts for all members of the Humifusa clade to ascertain whether geographic patterns are associated with differences in ploidy. We then related chromosomal data to observed morphological variability, polyploid formation, and consequently the evolutionary history of the clade. We counted chromosomes of 277 individuals from throughout the ranges of taxa included within the Humifusa clade, with emphasis placed on the widely distributed species, Opuntia humifusa (Raf.) Raf., 1820 s.l. and Opuntia macrorhiza Engelm., 1850 s.l. We also compiled previous counts made for species in the clade along with our new counts to plot geographic distributions of the polyploid and diploid taxa. A phylogeny using nuclear ribosomal ITS sequence data was reconstructed to determine whether ploidal variation is consistent with cladogenesis. We discovered that diploids of the Humifusa clade are restricted to the southeastern United States (U.S.), eastern Texas, and southeastern New Mexico. Polyploid members of the clade, however, are much more widely distributed, occurring as far north as the upper midwestern U.S. (e.g., Michigan, Minnesota, Wisconsin). Morphological differentiation, although sometimes cryptic, is commonly observed among diploid and polyploid cytotypes, and such morphological distinctions may be useful in diagnosing possible cryptic species. Certain polyploid populations of Opuntia humifusa s.l. and Opuntia macrorhiza s.l., however, exhibit introgressive morphological characters, complicating species delineations. Phylogenetically, the Humifusa clade forms two subclades that are distributed, respectively, in the southeastern U.S. (including all southeastern U.S. diploids, polyploid Opuntia abjecta Small, 1923, and polyploid Opuntia pusilla (Haw.) Haw., 1812) and the southwestern U.S. (including all southwestern U.S. diploids and polyploids). In addition, tetraploid Opuntia humifusa s.l., which occurs primarily in the eastern U.S., is resolved in the southwestern diploid clade instead of with the southeastern diploid clade that includes diploid Opuntia humifusa s.l. Our results not only provide evidence for the polyphyletic nature of Opuntia humifusa and Opuntia macrorhiza, suggesting that each of these represents more than one species, but also demonstrate the high frequency of polyploidy in the Humifusa clade and the major role that genome duplication has played in the diversification of this lineage of Opuntia s.s. Our data also suggest that the southeastern and southwestern U.S. may represent glacial refugia for diploid members of this clade and that the clade as a whole should be considered a mature polyploid species complex. Widespread polyploids are likely derivatives of secondary contact among southeastern and southwestern diploid taxa as a result of the expansion and contraction of suitable habitat during the Pleistocene following glacial and interglacial events.
Cactaceae, chromosome numbers, Opuntia humifusa, Opuntia macrorhiza, Pleistocene refugia, polyploid complex, polyploidy
Ploidy has a long tradition of utility for illuminating species boundaries, hybrid zones, and interspecific relationships among plants (e.g.,
The significance of polyploidy in plant evolution and speciation has long been recognized (
There are currently six species recognized in the Humifusa clade, Opuntia abjecta Small, 1923, Opuntia humifusa, Opuntia macrorhiza Engelm., 1850, Opuntia pottsii Salm-Dyck, 1849, Opuntia pusilla (Haw.) Haw., 1812, and Opuntia tortispina Engelm. & J.M. Bigelow, 1856 (Pinkava, 2003; LCM unpubl. data). The Humifusa clade is distributed widely from the western U.S. and northern Mexico (represented by Opuntia macrorhiza s.l., Opuntia pottsii, and Opuntia tortispina) and throughout the eastern U.S. including the upper Midwest (e.g., Michigan, Minnesota, Wisconsin) and southern Ontario (Benson, 1982; represented by Opuntia abjecta, Opuntia humifusa s.l., Opuntia macrorhiza s.l., and Opuntia pusilla).
Opuntia humifusa s.l. is composed of numerous morphological entities that have been recognized in certain taxonomic treatments as different species (see
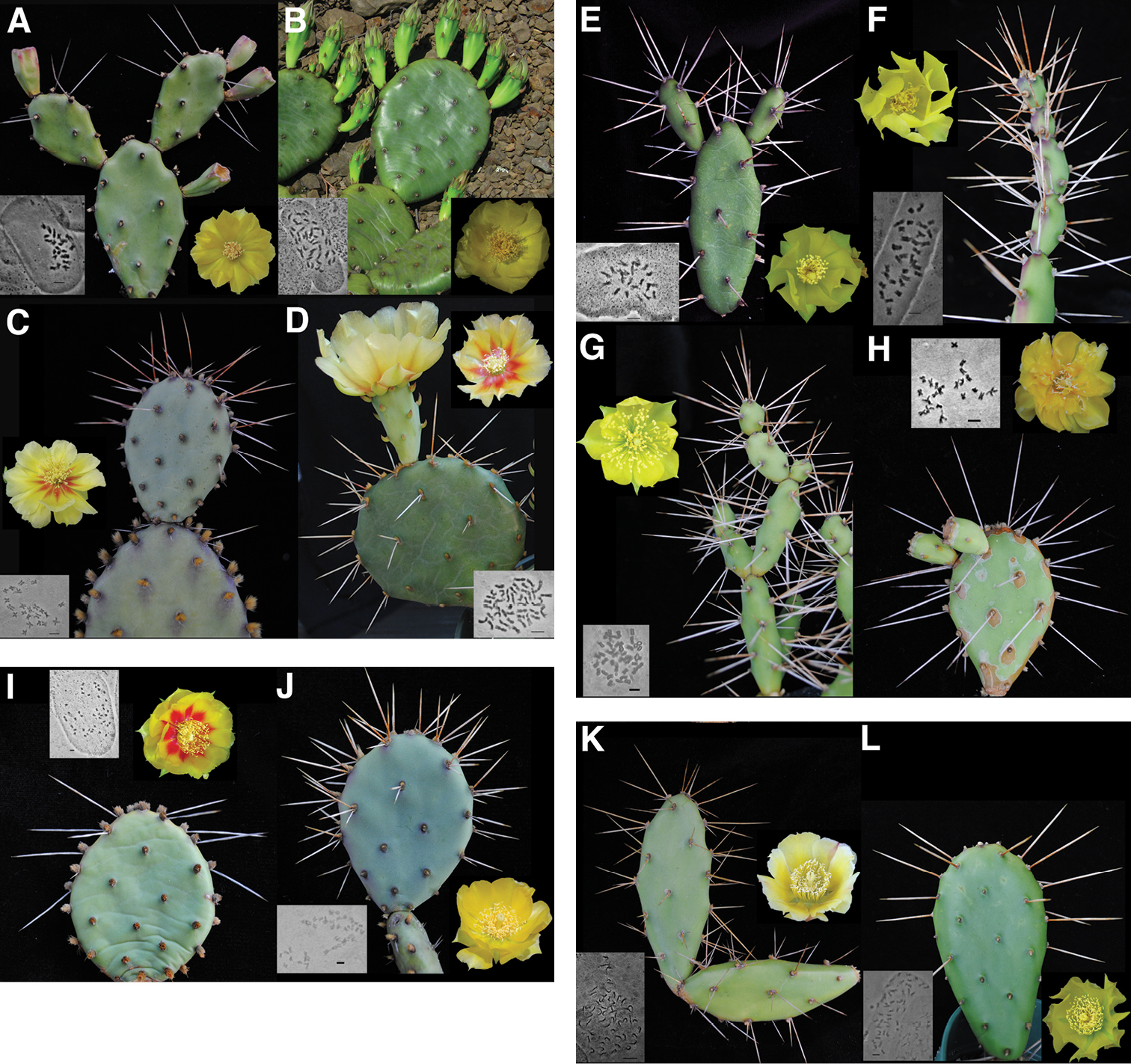
Selected taxa in the Humifusa clade with associated chromosome squashes A diploid Opuntia humifusa (Opuntia lata) LCM 4106 B tetraploid Opuntia humifusa s.s. LCM 3810 C diploid Opuntia macrorhiza (Opuntia xanthoglochia) LCM 1983 D tetraploid Opuntia macrorhiza LCM 3510 E diploid Opuntia pusilla LCM 753 F triploid Opuntia pusilla LCM 1033 G tetraploid Opuntia pusilla LCM 3700 H diploid Opuntia abjecta LCM 3908 I tetraploid Opuntia humifusa (Opuntia cespitosa) LCM 2610 J tetraploid Opuntia humifusa (Opuntia nemoralis) LCM 4204 K pentaploid Opuntia ochrocentra LCM 3907 and L tetraploid Opuntia humifusa (Opuntia pollardii) LCM 769. Bars on photomicrographs = 5μm.
Opuntia pusilla has been divided into several species: Opuntia drummondii Graham, 1841, Opuntia frustulenta Gibbes, 1858, Opuntia impedita Small, 1923, Opuntia pes-corvi LeConte, 1857, and Opuntia tracyi Britton, 1911 (
Contributing to the confusing taxonomic history of this clade is the high degree of morphological variation exhibited by most taxa, the lack of complete sampling throughout the range of the clade, the absence of cytological and phylogenetic evidence, reliance on poorly prepared and sparse herbarium collections (
Although chromosome counts have been reported for many of the Opuntia taxa from the southwestern U.S. and other areas (
Chromosome counts reported for species in the Humifusa clade do not encompass all of the taxa within the range of the clade nor the wide distributions exhibited by several of the more common species. To further our understanding of species complexes and the evolution of polyploids within those complexes, cytological data are needed from the entire distribution of a given species (
Here we present chromosome counts for all taxa considered to be part of the Opuntia humifusa complex and all taxa of the Humifusa clade (LCM, WSJ, PSS, DES, unpubl. data) and provide counts throughout most of the known ranges of all taxa to determine the geographic structure of ploidy and differences in ploidy among morphologically distinct taxa. We also reconstruct a phylogeny of diploid and polyploid members of the Humifusa clade based on nrITS data to investigate the relationship between geographic distribution and evolutionary relationships. We provide counts for another common species in the southeastern U.S., Opuntia stricta (Haw.) Haw., 1812, because it has been hypothesized to hybridize with members of the Humifusa clade (
Chromosome counts – Methods follow those of
Taxonomy – Taxa used for ploidy analysis are listed in Appendix 1. Species delimitations within Opuntia humifusa s.l. and Opuntia macrorhiza s.l. are problematic, so we recognize both Opuntia humifusa and Opuntia macrorhiza as broadly circumscribed (Table 1). Thus, we have arranged our counts of plants within these two species (see Appendix 1) according to their various segregates to determine whether the morphological variation of these segregate entities (Table 2) is correlated with cytotype and/or geographical and phylogenetic patterns.
Synonyms of Opuntia humifusa s.l. and Opuntia macrorhiza s.l. sampled during this study.
| Opuntia humifusa s.l. | Opuntia macrorhiza s.l. |
|---|---|
| Opuntia allairei | Opuntia fusco-atra |
| Opuntia ammophila | Opuntia grandiflora |
| Opuntia austrina | Opuntia xanthoglochia |
| Opuntia cespitosa | |
| Opuntia lata | |
| Opuntia nemoralis | |
| Opuntia pollardii |
Selected taxa of Opuntia humifusa s.l. and Opuntia macrorhiza s.l. with morphological characters and corresponding ploidy. Polyploids often exhibit characters from more than one diploid taxon or characters of other polyploids, although certain characters (e.g., red glochids) have not been observed in any diploids analyzed thus far.
| Taxon (ploidy) | Flower color | Cladode color | Spine barbedness/Cladode disarticulation | Glochid color |
|---|---|---|---|---|
| Opuntia ammophila (2x) | Yellow | Dark green | Not barbed/no | Stramineous |
| Opuntia austrina (2x) | Yellow | Dark green | Barbed/yes | Stramineous |
| Opuntia cespitosa (4x) | Red-centered | Glaucous green | Not barbed/no | Red |
| Opuntia lata (2x) | Yellow | Dark green | Barbed/yes | Stramineous |
| Opuntia humifusa (4x) | Yellow | Dark green | Not barbed/no | Stramineous |
| Opuntia macrorhiza (4x) | Red-centered | Glaucous green | Not barbed/no | Red/yellow |
| Opuntia nemoralis (4x) | Yellow | Glaucous green | Barbed/yes | Yellow |
| Opuntia pollardii (4x) | Yellow | Dark green | Barbed/yes | Stramineous |
| Opuntia xanthoglochia (2x) | Red-Centered | Glaucous green | Not barbed/no | Yellow |
Cytogeographic analysis – We mapped the localities for all of the individuals for which we determined ploidy (277 in number) and incorporated previous counts (n = 41) (
Phylogenetic analysis – We generated sequences from the nuclear ribosomal internal transcribed spacer (nrITS:
Taxa used in phylogenetic analyses of ITS sequence data given with their GenBank accession numbers.
| Accession | Locality | GenBank accession # |
|---|---|---|
| Opuntia basilaris (outgroup) | Inyo Co., CA R. Altig s.n. | JF786913 |
| Opuntia abjecta (2x) | Monroe Co., FL LCM 3908 | JF787021 |
| Opuntia abjecta (4x) | Monroe Co., FL LCM 3318 | JQ245716 |
| Opuntia ammophila (2x) | Marion Co., FL LCM 2826 | JF786904 |
| Opuntia austrina (2x) | Highlands Co., FL LCM 3450 | JF786911 |
| Opuntia cespitosa (4x) | Scott Co., MO LCM 2441 | JQ245717 |
| Opuntia humifusa (4x) | Warren Co., VA LCM 3800 | JQ245718 |
| Opuntia lata (2x) | Irvin Co., GA LCM 3785 | JF786949 |
| Opuntia macrorhiza (4x) | Kerr Co., TX LCM 3510 | JF786960 |
| Opuntia nemoralis (4x) | Garland Co., AR LCM 2196 | JQ245720 |
| Opuntia pusilla (2x) | Lowndes Co., MS LCM 843 | JQ245721 |
| Opuntia pusilla (3x) | Baldwin Co., AL LCM 1091 | JF786985 |
| Opuntia pusilla (4x) | Jackson Co., MS LCM 1920 | JF786986 |
| Opuntia tortispina (6x) | Hutchinson Co., TX LCM 3533 | JF787020 |
| Opuntia xanthoglochia (2x) | Bastrop Co., TX LCM 1982 | JQ245719 |
The base chromosome number for Cactaceae has been well established as x = 11 (
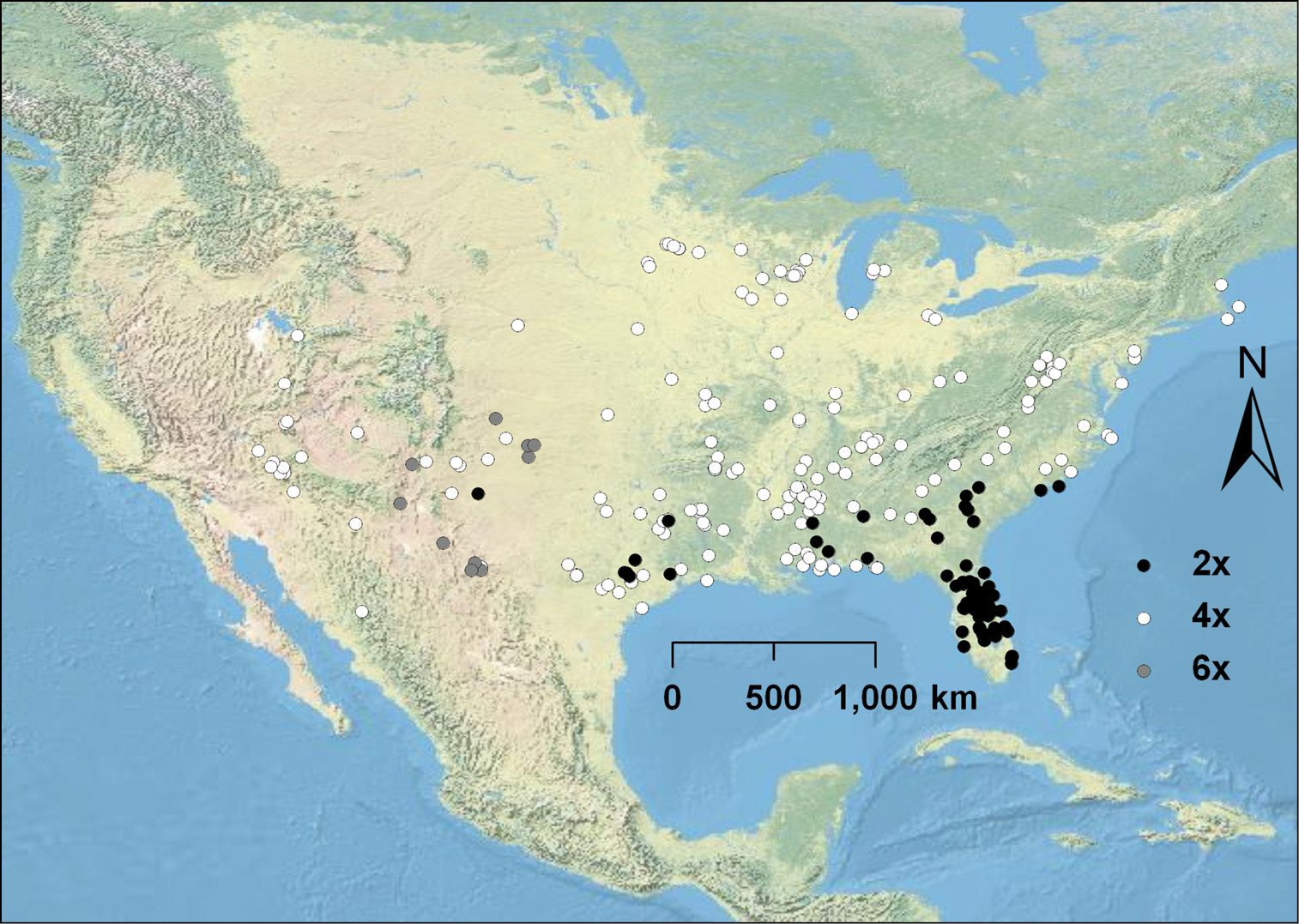
Cytogeography of Opuntia humifusa s.l., Opuntia macrorhiza s.l., Opuntia pottsii, and Opuntia tortispina. Diploids are represented with black circles, tetraploids by white circles, and hexaploids are represented by gray circles. Opuntia humifusa diploids are confined to the southeastern U.S., and Opuntia macrorhiza diploids are located in eastern Texas and southeastern New Mexico.
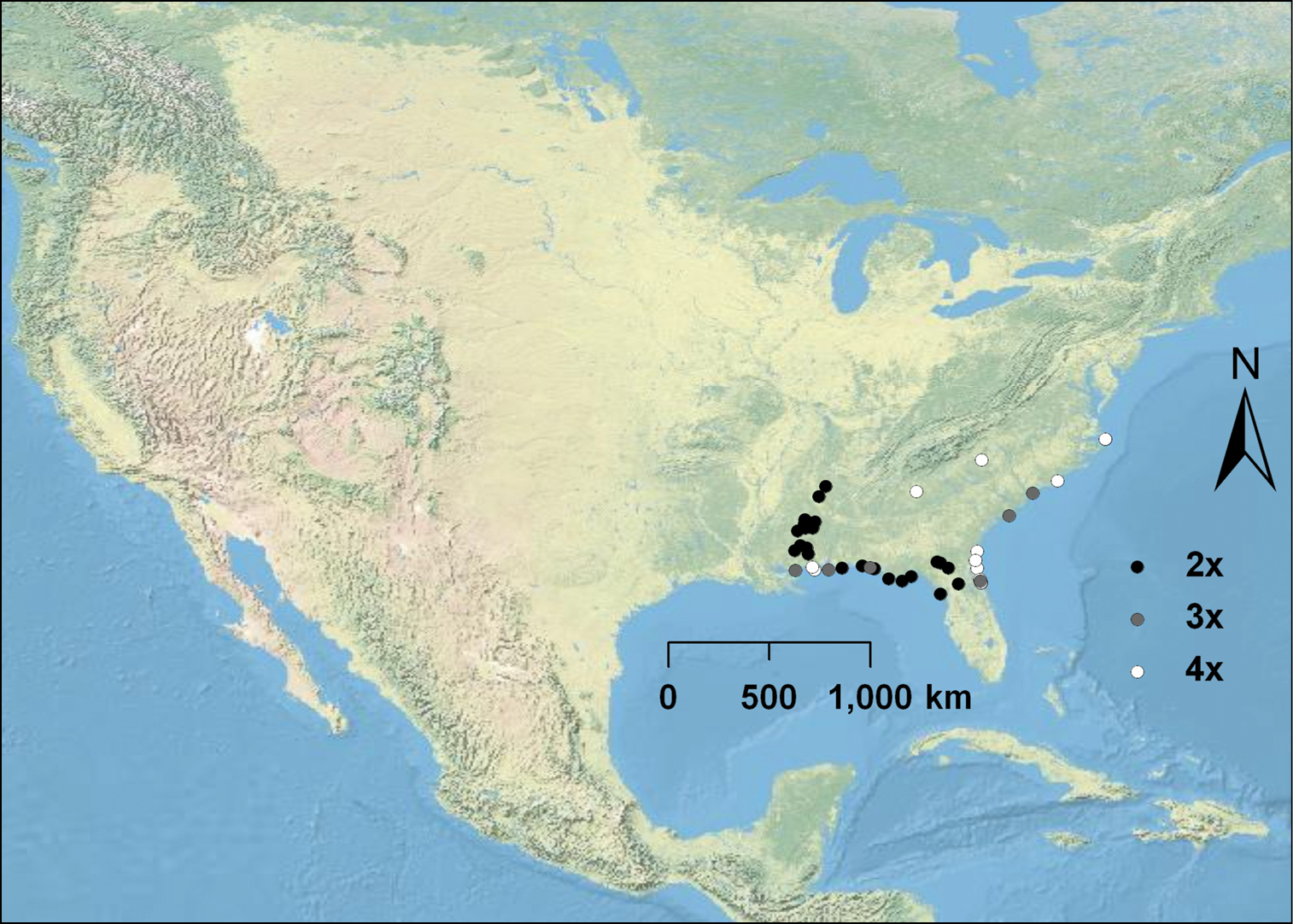
Diploid, triploid, and tetraploid populations of Opuntia pusilla were discovered (Fig. 1E-G) throughout its restricted range in the southeastern U.S. (Fig. 3). Interestingly, with the exception of two populations, polyploid individuals (3x and 4x) were mostly confined to the coastline, although diploid populations were much more widespread throughout the interior part of the distribution of the species (Fig. 3). Of the three examples of Opuntia abjecta sampled from the Florida Keys, one was diploid (Fig. 1H), and two were tetraploid. Opuntia tortispina (southwestern U.S.) was hexaploid in six and tetraploid in one of the populations examined (see Fig. 2 for hexaploid distribution).
Cytogeography of Opuntia pusilla. Diploids are represented by black circles, triploids by gray circles, and tetraploids by white circles. Note that most polyploids are restricted to coastal areas.
Individuals of Opuntia stricta sampled from the southeastern U.S. were all hexaploid. Samples included members of the taxa considered by some (
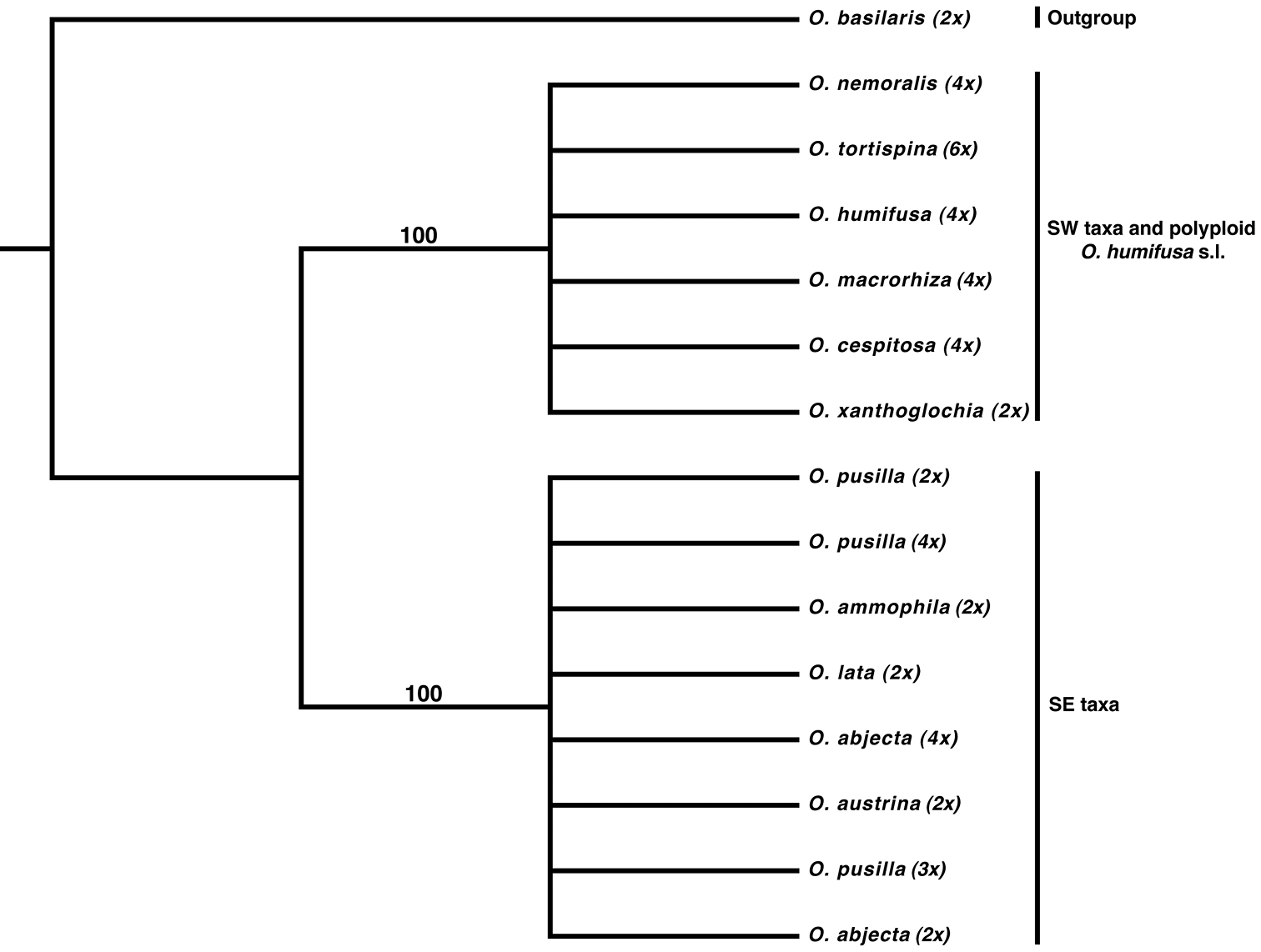
Maximum likelihood analysis of ITS data reveals that the Humifusa clade is made up of two well-supported subclades. One is restricted to the southeastern U.S. and includes polyploid members of Opuntia pusilla and Opuntia abjecta, and the other includes southwestern diploid Opuntia macrorhiza and all other polyploids pertaining to Opuntia humifusa s.l., Opuntia macrorhiza s.l., and Opuntia tortispina. There is no further resolution within the tree at the species level using ITS (Fig. 4). Species relationships within these two clades are further resolved with the addition of other loci (LCM unpubl. data), however, that is beyond the scope of this study.
Majority rule consensus topology from 10000 ML bootstrap pseudoreplicates using RAxML based on the nrITS region. The western diploid Opuntia macrorhiza s.l. (Opuntia xanthoglochia) forms a well-supported clade with polyploid Opuntia macrorhiza, Opuntia tortispina, and the eastern polyploid morphotypes of Opuntia humifusa s.l. (Opuntia cespitosa, Opuntia humifusa, and Opuntia nemoralis). The southeastern diploid morphotypes of Opuntia humifusa s.l. (Opuntia ammophila, Opuntia austrina, Opuntia lata) and diploid Opuntia abjecta and Opuntia pusilla form a well-supported clade with polyploid members of Opuntia pusilla and Opuntia abjecta.
Opuntia macrorhiza has only been recorded previously as tetraploid (
Diploid members of Opuntia humifusa s.l. (e.g., represented by the segregate taxa Opuntia ammophila Small, 1919, Opuntia austrina Small, 1903, Opuntia lata Small, 1919, in this study; see also Appendix 1) exhibit high levels of morphological variability but each is diagnosable morphologically, which suggests that these segregate taxa may need to be recognized at the species level. Likewise, diploid material of Opuntia macrorhiza s.l. from eastern Texas (e.g., Opuntia xanthoglochia Griffiths, 1910, in this study; see also Appendix 1) and southeastern New Mexico is morphologically distinct from tetraploid material of Opuntia macrorhiza s.l., which may also justify the recognition of Opuntia xanthoglochia and Opuntia macrorhiza as separate species.
Our hexaploid counts of Opuntia stricta are consistent with those of
Our three pentaploid counts of Opuntia ochrocentra support the proposed hybrid origin of this species between hexaploid Opuntia stricta (2n = 66) and diploid Opuntia abjecta (2n = 22) through unreduced gametes of Opuntia abjecta. Opuntia ochrocentra also exhibits intermediate morphological characters (e.g., growth form, spine characters) that further support its hybrid origin (LCM unpubl. data).
Diploid refugia and polyploid formation – Polyploidy is very common within the Humifusa clade, occurring in 66% of the samples reported here. Most researchers that have studied Opuntia cytologically have found polyploid taxa (e.g.,
The seemingly disjunct southeastern New Mexico diploid population of Opuntia macrorhiza s.l. may represent a mere extension of the eastern Texas diploid refugium, which has since been mostly replaced by polyploid taxa. Alternatively, a diploid extension may still exist but was not detected due to the lack of cytological data for populations from east Texas to southeastern New Mexico (Fig. 2). Diploid taxa of other clades (e.g., Opuntia polyacantha Haw. var. arenaria (Engelm.) Parfitt, 1819) are coincidentally found near the same region (Pinkava 2002, 2003), however, suggesting that a third diploid refugium, i.e., in southeastern New Mexico-western Texas, may need to be recognized.
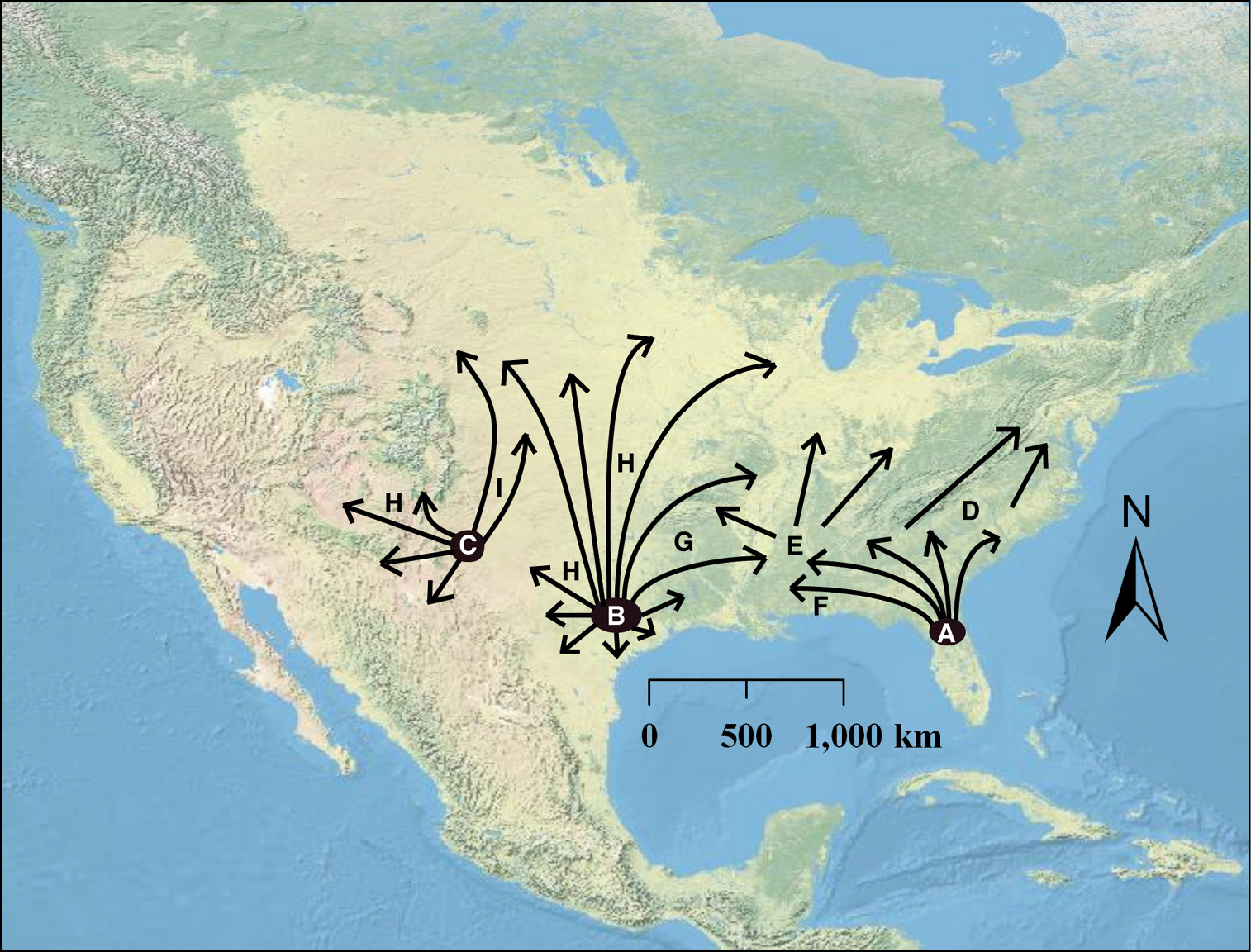
Pinkava (2003) suggested that an Opuntia humifusa - Opuntia macrorhiza - Opuntia pottsii complex originated along the east coast of the U.S. and spread westward to Arizona, where it came into contact and hybridized with Opuntia polyacantha and formed the mostly hexaploid Opuntia tortispina. From our data, this scenario is plausible in that Opuntia tortispina has morphological characters representative of both Opuntia polyacantha and Opuntia macrorhiza and is found where populations of diploid and tetraploid Opuntia macrorhiza s.l. and diploid Opuntia polyacantha come into contact. However, considering the two diploid refugia suggested by our analyses and what is known about the historical biogeography of the southeastern U.S. (e.g., Webb 1990), it is likely that the Humifusa clade originated in the southwestern U.S. and adjacent northern Mexico, then dispersed eastward into the southeastern U.S. The arid habitat along the coast of the Gulf of Mexico during the mid-Pliocene to early Pleistocene would have been interrupted during the mid-Pleistocene, creating the disjunction and promoting the genetic divergence among diploid populations we see today (Fig. 4). Taxa from these two diploid refugia would have come back into contact and formed the widely successful polyploids of the Midwest and eastern U.S. (Fig. 5). This scenario is further corroborated by phylogenetic analyses, where eastern U.S. polyploids of Opuntia humifusa s.l. are resolved in a clade with the southwestern diploid Opuntia macrorhiza (Fig. 4). The lower frequency of diploids encountered in western populations of the Humifusa clade also suggest that those diploid populations may be older (see
Hypothetical origin and subsequent dispersal of polyploid taxa from diploid refugia. Diploid refugia are represented by A southeastern Opuntia humifusa s.l. diploids B–C eastern Texas and southeastern New Mexico Opuntia macrorhiza s.l. diploids D–I represent polyploid formation where D represents Opuntia humifusa E represents Opuntia cespitosa F represents Opuntia pollardii G represents Opuntia nemoralis H represents tetraploid Opuntia macrorhiza (showing likely multiple formations), and I represents tetra- and hexaploid Opuntia tortispina.
The various morphotypes of tetraploid Opuntia macrorhiza in the western U.S. likely arose from southwestern diploid populations but subsequently spread in all directions after formation. Tetraploid Opuntia macrorhiza appears to have arisen numerous times, given that several morphotypes exist throughout its range. However, only two diploid morphotypes are known to exist (eastern Texas and southeastern New Mexico), suggesting that other ancestral diploids may have since gone extinct or have not yet been found, or that polyploid taxa exhibiting unique, derived characters were partly responsible for the origin of certain morphotypes, which have no diploid counterparts.
Many polyploid populations of Opuntia humifusa s.l. and Opuntia macrorhiza s.l., especially in the eastern U.S., are largely isolated from one another and from diploid populations, suggesting that polyploid formation is not ongoing, at least on such a large scale as during the Pleistocene or immediately after the last glacial maximum. In contrast, polyploids in Opuntia pusilla are mostly sympatric with diploids in the Gulf of Mexico region and are represented by triploids and tetraploids. Polyploids of Opuntia pusilla also do not share the wide geographic distribution of those polyploids derived from Opuntia humifusa s.l. and Opuntia macrorhiza s.l. These observations suggest that the polyploids of Opuntia pusilla may have formed only recently, do not share comparable dispersal agents, or lack the obvious adaptive advantages of those polyploids derived from Opuntia humifusa s.l. and Opuntia macrorhiza s.l.
Many polyploid populations of Opuntia humifusa s.l. and Opuntia macrorhiza s.l.occupy northerly distributions and thus have a very high tolerance to cold temperatures. The hexaploid Opuntia fragilis (Nutt.) Haw., 1819 (not in the Humifusa clade) similarly inhabits areas of northern North America (
Agamospermy – The tetraploid Opuntia cespitosa (an entity within Opuntia humifusa s.l.; see Table 1) produces viable seed in the absence of outcrossing (Majure pers. obsv.), so this taxon is either self-compatible, which is common in Cactaceae (
Autopolyploidy vs. Allopolyploidy – The mechanism by which Opuntia polyploids are formed (auto- vs. allopolyploidy) is unclear. Unreduced gametes have frequently been found in meiotic analyses of Cactaceae (e.g.,
Opuntia humifusa as currently circumscribed consists of numerous morphological entities, which are either diploid or tetraploid; those populations differing in ploidy are generally geographically well separated from one another. It is evident from our phylogenetic analysis (Fig. 4) that Opuntia humifusa is polyphyletic. Considering morphological and genetic data, it is likely that tetraploid Opuntia humifusa is of allopolyploid origin. However, the pattern in Opuntia pusilla is different, with populations of diploids found in close proximity to populations of triploids and tetraploids (Fig. 3). This evidence, plus morphological similarity among ploidal levels, suggests possible formation of autopolyploids. This same pattern is seen in other autopolyploid taxa (
Morphological correlations with polyploids – Some polyploid taxa in the Humifusa clade share morphological characters with diploids and other polyploids, suggesting that they may be derived from hybridization (Table 2). Opuntia nemoralis Griffiths, 1913, (Fig. 1J; an entity within Opuntia humifusa s.l.; see Table 1) shares spine color and orientation, cladode color, and glochid color of tetraploid Opuntia macrorhiza (from Arkansas), although, it possesses small and easily disarticulating cladodes, retrorsely-barbed spines, and the pile forming growth form and yellow flowers of Opuntia pusilla (Fig. 1E-G). Opuntia cespitosa (Table 1), as mentioned above, exhibits the red-centered flowers, glaucous-gray cladodes, and dark glochids (Fig. 1I) of tetraploid Opuntia macrorhiza (Fig. 1D), as well as the spine characters of diploid Opuntia humifusa s.l. (= Opuntia ammophila, Opuntia austrina, Opuntia lata; Table 2).
Throughout the distribution of the most common polyploid taxa, there also are polyploid populations that appear to be introgessive products of hybridization with other polyploids. For instance, in Michigan, Wisconsin, and western Illinois, certain populations display characters of both Opuntia cespitosa and tetraploid Opuntia macrorhiza (see
In the eastern U.S., most populations are represented by only one morphotype and thus appear to be morphologically stable (except for typically variable characters such as spine number; see
Members of the Humifusa clade are found throughout most of the continental U.S., with no obvious breaks or disjunctions in distribution patterns until detailed analyses of chromosome number were carried out. Our analyses indicate that diploid taxa in the Humifusa clade are presently confined to the southwestern and the southeastern U.S., which likely represent Pleistocene refugia for these taxa. Polyploid taxa of Opuntia humifusa s.l. and Opuntia macrorhiza s.l. were likely formed when diploids from these two refugia came into contact during interglacial cycles of the Pleistocene. This scenario is supported further by phylogenetic analyses, in which two clades correspond to these two diploid refugia, and polyploid taxa are found in either clade. Polyploid taxa likely also contributed to the diversity of polyploid morphotypes through secondary contact and introgression with other polyploids. After the end of the last glacial maximum, open niches would have been readily available for colonization by polyploid taxa produced towards the leading edge of the expansion and distribution of the Humifusa clade. These polyploids subsequently dispersed throughout most of the continent and occupied all suitable habitats available after glacial retreat, accounting for the distribution that we see today. Distributional success was enabled by the extreme cold tolerance displayed by many of the polyploid taxa, which allowed them to colonize more northern areas presumably unsuitable for diploid taxa.
We would like to thank M. Arakaki and V. Suarez for demonstrating some of their chromosome counting methods to L.C. Majure. We thank V. Doyle, P. Oudemans (NYBG), J. G. Hill (MSU), H. Sullivan, T. Mann (MS Museum of Natural Science), M.J. Moore (Oberlin College), B. Nichols (NH Natural Heritage Bureau), K.D. Philley (MS College), D.J. Pinkava and M. Baker (ASU), C. Reid (LA Natural Heritage Program), E. Ribbens (WIU), B. Wellard, T. Frates, D. Woodruff (Utah Native Plant Society), G.P. Johnson, T. Witsell (AR Natural Heritage Program), T. Harrison (Westminster College), B. Snow, and K. Sauby (UF) for some specimens used in counts, Key Deer National Wildlife Refuge for access to their property, B. Connolly (MA Natural Heritage Program) for help with permits, F. Axelrod (UPR), T.C. Majure, C. Doffitt, G.N. Ervin (MSU), and B. Patenge for help with fieldwork, and M. Pajuelo (UF) for help with fieldwork and illustrations. We also thank two anonymous reviewers for comments on an earlier version of this manuscript. This work was supported in part by funding from the USGS Biological Resources Discipline (#04HQAG0135) to Gary N. Ervin, a New England Botanical Club graduate research grant, the American Society of Plant Taxonomists Shirley and Alan Graham student research award, a Cactus and Succulent Society of America research grant, the Florida Plant Conservation Program, and NSF Dissertation Improvement Grant (DEB-1011270). Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund.
Currently recognized Opuntia species investigated are listed (1-6). Synonyms of recognized species (sensu Benson 1982, Pinkava 2003, and Powell et al. 2008 in part; see Table 1) and their respective ploidy are given below the recognized species name. Recognized species are split by ploidy, where species have more than one cytotype. Their somatic chromosome number is given along with locality, collector, and repository according to Index Herbariorum (Thiers 2011). Taxa counted for the first time or cytotypes not previously recorded for a species are delimited with an asterisk (*). All counts were made by L.C. Majure.
1) Opuntia abjecta Small
* Opuntia abjecta Small; 2n = 22 Florida, Monroe Co., LCM 3908 (FLAS). * Opuntia abjecta Small; 2n = 44, Florida, Monroe Co., LCM 3318 (FLAS), Monroe Co., KS s.n. (FLAS).
2) Opuntia humifusa (Raf.) Raf.
Opuntia humifusa (2x) taxa: Opuntia ammophila Small; 2n = 22, Florida, Brevard Co., LCM 2087 (MISSA), Broward Co., KS 62 (FLAS), Flagler Co., LCM 3222 (FLAS), Indian River Co., LCM 4182 (FLAS), Indian River Co., LCM 4183 (FLAS), Indian River Co., LCM 4184 (FLAS), Lake Co., LCM 3246 (FLAS), Lake Co., LCM 4093 (FLAS), Marion Co., LCM 2753 (FLAS), Marion Co., LCM 2754 (FLAS), Marion Co., LCM 2826 (FLAS), Marion Co., LCM 3247 (FLAS), Okeechobee Co., LCM 4185 (FLAS), Okeechobee Co., LCM 4186 (FLAS), Orange Co., LCM 2086 (MISSA), Orange Co., LCM 3962 (FLAS), Osceola Co., LCM 3702 (FLAS), Osceola Co., LCM 4181 (FLAS), Osceola Co., LCM 4189 (FLAS), Putnam Co., LCM 3248 (FLAS), St. Johns Co., K.S. s.n. (FLAS), St. Lucie Co., LCM 3704 (FLAS), St. Lucie Co., LCM 3705 (FLAS), St. Lucie Co., LCM 3708 (FLAS), Seminole Co., LCM 2085 (MISSA), Volusia Co., LCM 3224 (FLAS), Volusia Co., LCM 3232 (FLAS). Opuntia austrina Small; 2n = 22, Florida, Charlotte Co., KS 45 (FLAS), Highlands Co., FL KS 64 (FLAS), Highlands Co., LCM 3450 (FLAS), Highlands Co., LCM 3975 (FLAS), Highlands Co., LCM 3976 (FLAS), Highlands Co., LCM 3978 (FLAS), Okeechobee Co., KS 29 (FLAS), Okeechobee Co., KS 42 (FLAS), Palm Beach Co., LCM 3970 (FLAS), Palm Beach Co., LCM 3973 (FLAS), Polk Co., KS s.n. (FLAS), Polk Co., LCM 3979 (FLAS). Opuntia lata Small; 2n = 22, Alabama, Autauga Co., LCM 2043 (MISSA), Mobile Co., LCM 4194 (FLAS), Florida, Alachua Co., LCM 3991 (FLAS), Alachua Co., LCM 4061 (FLAS), Alachua Co., LCM 4064 (FLAS), Hernando Co., LCM 3948 (FLAS), Highlands Co., LCM 3977 (FLAS), Lafayette Co., LCM 2795 (FLAS), Lake Co., KS 15 (FLAS), Lake Co., LCM 4117 (FLAS), Levy Co., LCM 3645 (FLAS), Manatee Co., LCM 4065 (FLAS), Okaloosa Co., LCM 3954 (FLAS), Okeechobee Co., LCM 4187 (FLAS), Okeechobee Co., LCM 4188 (FLAS), Orange Co., LCM 4174 (FLAS), Palm Beach Co., LCM 3971 (FLAS), Putnam Co., LCM 4106 (FLAS), Sumter Co., LCM 3238 (FLAS), Sumter Co., LCM 4066 (FLAS), Georgia, Charlton Co., LCM 4190 (FLAS), Crawford Co., JH s.n. (FLAS), Irwin Co., LCM 3785 (FLAS), Perry Co., LCM 3786 (FLAS), Tatnall Co., JH s.n. (FLAS), Mississippi, Newton Co., LCM 938 (MISSA), Wayne Co., LCM 1290 (MISSA), South Carolina, Aiken Co., LCM 3588 (FLAS), Horry Co., LCM 3832 (FLAS).
Opuntia humifusa (4x) taxa: *Opuntia allairei Griffiths; 2n = 44, Texas, Liberty Co., LCM 3504 (FLAS). *Opuntia cespitosa Raf.; 2n = 44, Alabama, Bibb Co., LCM 2042 (MISSA), Colbert Co., LCM 2610 (MISSA), Lawrence Co., LCM 2609 (MISSA), Arkansas, Garland Co., LCM 2198 (FLAS), Garland Co., LCM 4203 (FLAS), Garland Co., LCM 4205 (FLAS), Saline Co., LCM 2194 (MISSA), Yell Co., GPJ s.n. (FLAS), Illinois, Cass Co., IL ER s.n. (FLAS), Jo Daviess Co., IL ER s.n. (FLAS), Kentucky, Anderson Co., LCM 3276 (FLAS), Louisiana, Caddo Parish, LCM 4200 (FLAS), Caddo Parish, LCM 4201 (FLAS), Caddo Parish, LCM 4202 (FLAS), Massachusetts, Dukes Co., BC s.n. (FLAS), Mississippi, Lee Co., MS JH s.n. (FLAS), Lowndes Co., LCM 755 (MISSA), Oktibbeha Co., LCM 1380 (MISSA), Scott Co., LCM 2563 (MISSA), Tennessee, Bledsoe Co., LCM 1938 (MISSA), Cannon Co., LCM 2072 (MISSA), Davidson Co., JH s.n. (FLAS), Fayette Co., LCM 1956 (MISSA; note Opuntia cf. cespitosa), Fayette Co., JH s.n. (note Opuntia cf. cespitosa FLAS), Franklin Co., BLS 2061 (FLAS), Lewis Co., JH s.n. (FLAS), Marshall Co., JH s.n. (FLAS), Rutherford Co., JH s.n. (FLAS), Texas, Lamar Co., BS 2069 (FLAS), Virginia, Fredrick Co., LCM 3806 (FLAS). Opuntia humifusa (Raf.) Raf.; 2n = 44, Alabama, Marion Co., AL JH s.n. (FLAS), Delaware, Sussex Co., LCM 3824 (FLAS), Georgia, Dekalb Co., GA LCM 3787 (FLAS), Jackson Co., LCM 3789 (FLAS), Marion Co., JH s.n. (FLAS), Maryland, Alleghany Co., LCM 3810 (FLAS), Massachusetts, Barnstable Co., MA LCM 3814 (FLAS), Mississippi, Calhoun Co., MS JH s.n. (FLAS), Carroll Co, LCM 799 (MISSA), Choctaw Co., KP 499 (MMNS), Grenada Co., LCM 1833 (MISSA), Marion Co., JH s.n. (FLAS), Marshall Co., LCM 1293 (MISSA), Montgomery Co., LCM 768 (MISSA), Stone Co., TM s.n. (FLAS), Webster Co., KP 498 (MMNS), Yalobusha Co., LCM 767 (MISSA), New Hampshire, Rockingham Co., BN s.n. (FLAS), New Jersey, Atlantic Co., VD s.n. (FLAS), Burlington Co., LCM 3821 (FLAS), North Carolina, Bladen Co., JH s.n. (FLAS), Currituck Co., LCM 3825 (FLAS), Dare Co., LCM 3827 (FLAS), Onslow Co., LCM 3829 (FLAS), Rowan Co., LCM 3793 (FLAS), Surry Co., JH s.n. (FLAS), South Carolina, Pickens Co., LCM 3790 (FLAS), York Co., LCM 3791 (FLAS), Virginia, Fredrick Co., LCM 3807 (FLAS), Page Co., LCM 3799 (FLAS), Warren Co., LCM 3800 (FLAS), West Virginia, Hampshire Co., LCM 3808 (FLAS), Mineral Co., LCM 3809 (FLAS), Pendleton Co., ER s.n. (FLAS). *Opuntia nemoralis Griffiths, 2n = 44, Arkansas, Garland Co., LCM 2192 (MISSA), Garland Co., LCM 2196 (MISSA), Garland Co., LCM 4204 (FLAS); Louisiana, Beauregard Parish, CR s.n. (FLAS), Cameron Parish, LCM 4196 (FLAS), DeSoto Parish, LCM 4198 (FLAS), Red River Parish, LCM 4199 (FLAS), Winn Parish, BLS 2053 (FLAS). *Opuntia cf. nemoralis Griffiths, 2n = 44, Arkansas, Pulaski Co., BLS 2131 (FLAS), Yell Co., TW s.n. (FLAS). *Opuntia pollardii Britton & Rose; 2n = 44, Alabama, Baldwin Co., LCM 1082 (MISSA), Florida, Santa Rosa Co., LCM 1075 (MISSA), Walton Co., LCM 1067 (MISSA), Walton Co., LCM 1070 (MISSA), Louisiana, Washington Parish, CR s.n. (FLAS), Mississippi, Forrest Co., LCM 806 (MISSA), Hancock Co., LCM 748 (MISSA), Jackson Co., LCM 1921 (MISSA), Jackson Co., LCM 1297 (MISSA), Jackson Co., LCM 4057 (FLAS), Jackson Co., LCM s.n. (MMNS), Neshoba Co., LCM 1201 (MISSA), Noxubee Co., LCM 1156 (MISSA), Stone Co., TM s.n. (FLAS), Winston Co., LCM 769 (MISSA).
3) Opuntia macrorhiza Engelm.
Opuntia macrorhiza (2x) taxa: * Opuntia xanthoglochia Griffiths, 2n = 22, Texas, Bastrop Co., LCM 1982 (MISSA), Bastrop Co., MJM 949 (FLAS), Fayette Co., LCM 1983 (MISSA), Harris Co., BLS 2089 (FLAS), Milam Co., TX MJM 947 (FLAS), Smith Co., BLS 2082 (FLAS).
Opuntia macrorhiza (4x) taxa: *Opuntia fusco-atra Engelm.; 2n = 44, Texas, Fayette Co., LCM 3505 (FLAS). *Opuntia grandiflora Engelm.; 2n = 44, Arkansas, Miller Co., BLS 2062 (FLAS), Mississippi, Bolivar Co., LCM 1680 (MISSA), Holmes Co., HS s.n. (FLAS), Yazoo Co., LCM 2366 (MISSA), Texas, Anderson Co., BLS 2077 (FLAS), Austin Co., BLS 2091 (FLAS), Henderson Co., BLS 2081 (FLAS), Jack Co., LCM 3536 (FLAS), Leon Co., BLS 2074 (FLAS), Marion Co., BLS 2086 (FLAS), Smith Co., LCM 3540 (FLAS), Van Zandt Co., BLS 2083 (FLAS). Opuntia macrorhiza Engelm., 2n = 44, Arkansas, Nevada Co., BLS 2130 (FLAS), Newton Co., MC s.n. (FLAS), Pulaski Co., LCM 4206 (FLAS), Arizona, Coconino, TH s.n. (FLAS), Coconino, BW s.n. (FLAS), Nebraska, Keith Co., NE ER s.n. (FLAS), Lancaster Co., TH s.n. (FLAS), New Mexico, Torrance Co., LCM 3530 (FLAS), Texas, Calhoun Co., TX MJM 962 (FLAS), Dallas Co., LCM 3539 (FLAS), Gonzales Co., MJM 958 (FLAS), Kimble Co., LCM 3511 (FLAS), Kerr Co., LCM 3508 (FLAS), Kerr Co., LCM 3510 (FLAS), Palo Pinto Co., LCM 3537 (FLAS), Utah, Salt Lake Co., TH s.n. (FLAS), Sevier Co., TH s.n. (FLAS).
4) Opuntia pusilla (Haw.) Haw.
* Opuntia pusilla, 2n = 22, Alabama, Lamar Co., JH s.n. (FLAS), Florida, Alachua Co., LCM 4003 (FLAS), Bay Co., KS 307 (FLAS), Bay Co., KS 309 (FLAS), Columbia Co., LCM 4191 (FLAS), Escambia Co., KS 328 (FLAS), Franklin Co., KS 301 (FLAS), Franklin Co., KS 330 (FLAS), Gulf Co., KS 325 (FLAS), Hamilton Co., LCM 4192 (FLAS), Hamilton Co., FL LCM 4193 (FLAS), Levy Co., LCM 2819 (FLAS), Mississippi, Clarke Co., LCM 1270 (MISSA), Forrest Co., LCM 756 (MISSA), Jasper Co., LCM 766 (MISSA), Lamar Co., LCM 1548 (MISSA), Lauderdale Co., LCM 2094 (MISSA), Lauderdale Co., LCM 3919 (MISSA), Lowndes Co., LCM 843 (MISSA), Newton Co., LCM 828 (MISSA), Newton Co., LCM 937 (MISSA), Newton Co., LCM 4211 (FLAS), Perry Co., LCM 757 (MISSA), Smith Co., LCM 753 (MISSA), Wayne Co., TM s.n. (FLAS), Wayne Co., TM s.n. (FLAS). * Opuntia pusilla, 2n = 33, Alabama, Baldwin Co., LCM 1091 (MISSA), Florida, Flagler Co., LCM 3221 (FLAS), St. Johns Co., LCM 3219 (FLAS), Walton Co., LCM 1066 (MISSA), Mississippi, Hancock Co., LCM 1033 (MISSA), South Carolina, Horry Co., JH s.n. (FLAS), Horry Co., LCM 3833 (FLAS). Opuntia pusilla, 2n = 44, Florida, Duval Co., LCM 3700 (FLAS), Nassau Co., CJ s.n. (FLAS), St. Johns Co., LCM 3218 (FLAS), St. John’s Co., KS 9.4.10 (FLAS), Georgia, Dekalb Co., LCM 3788 (FLAS), Glynn Co., TM s.n. (FLAS), Mississippi, Jackson Co., LCM 955 (MISSA), Jackson Co., LCM 1920 (MISSA), North Carolina, Dare Co., LCM 3828 (FLAS), Dare Co., LCM 3836 (FLAS), New Hanover Co., LCM 3830 (FLAS), South Carolina, York Co., LCM 3792 (FLAS).
5a) Opuntia stricta (Haw.) Haw.
Opuntia dillenii (Ker-Gawl.) Haw., 2n = 66, Florida, Charlotte Co., LCM 3949 (FLAS), Flagler Co., LCM 3220 (FLAS), Monroe Co., LCM 3319 (FLAS), Hillsborough Co., LCM 3952 (FLAS), Puerto Rico, Cabo Rojo, LCM 3843 (FLAS). Opuntia stricta (Haw.) Haw., 2n = 66, Alabama, Mobile Co., LCM 823 (MISSA), Florida, Clay Co., LCM 3701 (FLAS), Levy Co., LCM 2820 (FLAS), Monroe Co., LCM 3320 (FLAS), St. Johns Co., LCM 3217 (FLAS), Seminole Co., LCM 2083 (MISSA), Mississippi, Jackson Co., LCM 1922 (MISSA).
5b) Putative hybrids involving Opuntia stricta.
Opuntia alta Griffiths 2n = 66, Louisiana, Cameron Parish, LCM 4195 (FLAS), LaFourche Parish, CR s.n. (FLAS). * Opuntia ochrocentra Small, 2n = 55, Florida, Monroe Co., LCM 3907 (FLAS), Monroe Co., LCM 3968 (FLAS), Monroe Co., LCM 3969 (FLAS).
6) Opuntia tortispina Engelm. & J.M. Bigelow.
Opuntia tortispina, 2n = 44, New Mexico, Quay Co., LCM 3531 (FLAS), Opuntia tortispina, 2n = 66, New Mexico, Benalillo Co., LCM 3528 (FLAS), Sierra Co., LCM 3521 (FLAS), Oklahoma, Cimarron Co., ER s.n. (FLAS), Texas, Carson Co., LCM 3532 (FLAS), Hutchinson Co., LCM 3533 (FLAS), Hutchinson Co., LCM 3535 (FLAS).