(C) 2012 Emanuel R. M. Martinez. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Cytogenetic analyses were accomplished in two populations of Astyanax altiparanae Garutti & Britzki, 2000 and one population of Hyphessobrycon eques Steindachner, 1882, considered incertae sedis in Characidae family. Two populations of Astyanax altiparanae (Mogi-Guaçu and Tietê rivers) presented 2n=50, with the same karyotype formula: 6M+12SM+20ST+12A (FN=88). Hyphessobrycon eques from Capivara river presented 2n=52 and karyotype formula 14M+16SM+4ST+18A (FN=86). In each karyotype, the nucleolus organizer regions were detected at the end of the short arm of a single medium-sized subtelocentric chromosome. The Chromomycin A3 (CMA3) marking is coincident for the NORs in chromosomes of the two species and present additionally in two different chromosomes of Astyanax altiparanae thus showinginterpopulation differences in this species. In Hyphessobrycon eques, weak heterochromatic blocks in the position of centromeres and telomeres of most chromosomes and negative C-banding for the NOR bearing chromosome were visualized. The obtained results contribute both to the understanding of karyotype evolution of these species and to the clarifying their phylogenetic relationships.
Ag-NOR, Chromomycin A3, chromosomes, evolution, Neotropical fish
The neotropical freshwater ichthyofauna is quite rich, including 71 families and more than 4, 500 species known to be valid, according to the latest surveys (
Astyanax Baird and Girard, 1854and Hyphessobrycon Eigenmann, 1908 are genera from family Characidae with wide distribution throughout Central and South America, and previously placed in subfamily Tetragonopterinae (
Astyanax includes about 100 species, commonly known as lambari and piaba (
The genus Hyphessobrycon with approximately 90 species is characterized by an interrupted lateral line, reaching up to 60 mm in total length, and some species present a remarkable color that may interest the aquarists (
Cytogenetic analysis of representatives of the genus Astyanax reveals that diploid numbers range from 2n=36 in Astyanax schubarti Britski, 1964 (
Summary of cytogenetic data from Brazilian populations of Astyanax altiparanae and Hyphessobrycon spp. 2n - diploid number; M - metacentric; SM - submetacentric; ST - subtelocentric; A - acrocentric; FN = fundamental number; n-NORs - number of chromosomes with silver stained nucleolar organizer regions. * - cited as Astyanax bimaculatus Linnaeus, 1758. Location’s list follows a geographical order.
| Species | Location | 2n | Karyotype | FN | n-NORs | Reference |
|---|---|---|---|---|---|---|
| Astyanax altiparanae Garutti & Britzki, 2000 | Iguaçu river, Curitiba, PR (Iguaçu river basin) | 50 | 6M+30SM+8ST+6A | 94 | 2 |
|
| Jordão river, Manguerinha, PR (Iguaçu river basin) | 50 | 6M+28SM+8ST+8A | 92 | 2-4 |
|
|
| Índios river, Cianorte, PR (Ivaí river basin) | 50 | 6M+30SM+4ST+10A | 90 | 10 |
|
|
| Tatupeba river, Maringá, PR (Ivaí river basin) | 50 | 6M+26SM+6ST+12A | 88 | 3 |
|
|
| *Meia Ponte river, Goiânia, GO (Meia Ponte river basin) | 50 | 26M+24A | 76 | - |
|
|
| Feijão stream, São Carlos, SP (Mogi-Guaçu river basin) | 50 | 6M+30SM+8ST+6A | 94 | 1-3 |
|
|
| *Mogi-Guaçu river, Pirassununga, SP (Mogi-Guaçu river basin) | 50 | 10M+24SM+4ST+12A | 88 | - |
|
|
| Mogi-Guaçu river, Pirassununga, SP (Mogi-Guaçu river basin) | 50 | 6M+12SM+20ST+12A | 88 | 2 | Present study | |
| Paraná river, Porto Rico, PR (Paraná river basin) | 50 | 6M+26SM+6ST+12A | 88 | 2 |
|
|
| Claro river, Tamarana, PR (Paranapanema river basin) | 50 | 10M+26SM+4ST+10A | 90 | 1-4 |
|
|
| Claro river, Tamarana, PR (Paranapanema river basin) | 50 | 10M+24SM+4ST+12A | 88 | 1-4 |
|
|
| Claro river, Tamarana, PR (Paranapanema river basin) | 50 | 10M+22SM+4ST+14A | 86 | 1-4 |
|
|
| Paranapanema river, Salto Grande, SP (Paranapanema river basin) | 50 | 10M+22SM+6ST+12A | 88 | - |
|
|
| Keçaba river, Maringá, PR (Pirapó river basin) | 50 | 6M+26SM+6ST+12A | 88 | 1 |
|
|
| Astyanax altiparanae Garutti & Britzki, 2000 | Maringá river, Maringá, PR (Pirapó river basin) | 50 | 6M+26SM+6ST+12A | 88 | 3 |
|
| *São Francisco river, MG (São Francisco river basin) | 50 | - | - | - |
|
|
| Tibagi river, Ponta Grossa, PR (Tibagi river basin) | 50 | 6M+28SM+8ST+8A | 92 | 2-3 |
|
|
| *Jurumirim river, SP (Tietê river basin) | 50 | - | - | - |
|
|
| Pântano stream, São Carlos, SP (Tietê river basin) | 50 | 6M+28SM+4ST+12A | 88 | 1-2 |
|
|
| Tietê river, Penápolis, SP (Tietê river basin) | 50 | 6M+12SM+20ST+12A | 88 | 2 | Present study | |
| Hyphessobrycon anisitsi Eigenmann, 1907 | Piracuama river (Paraíba do Sul river basin) | 50 | 6M+16SM+12ST+16A | 84 | 4 |
|
| Hyphessobrycon anisitsi Eigenmann, 1907 | Perdizes stream (Paraná river basin) | 50 | 6M+16SM+12ST+16A | 84 | 3 |
|
| Hyphessobrycon flammeus Myers, 1924 | Paraná river (Paraná river basin) | 52 | 18M, SM+32ST+2A | 102 | - |
|
| Hyphessobrycon reticulatus Ellis, 1911 | Juquiá river, São Lourenço da Serra, SP (Paraná river basin) | 50 | 14M+20SM+16ST | 100 | 2 |
|
| Hyphessobrycon scholzei Ahl, 1937 | Perdizes stream (Paraná river basin) | 50 | 8M+20SM+8ST+14A | 86 | - |
|
| Hyphessobrycon griemi Hoedeman, 1957 | Itimirim river, Iguape, SP, Iguape river basin (Ribeira river basin) | 48 | - | - | - |
|
| Hyphessobrycon herbertaxelrodi Géry, 1961 | Itimirim river, Iguape, SP, Iguape river basin (Ribeira river basin) | 52 | 10M, SM+42ST, A | - | - |
|
| Hyphessobrycon reticulatus Ellis, 1911 | Itimirim river, Iguape, SP, Iguape river basin (Ribeira river basin) | 50 | - | - | - |
|
| Hyphessobrycon eques Steindchner, 1882 | Capivara river, Botucatu, SP (Tietê river basin) | 52 | 14M+16SM+4ST+18A | 86 | 2 | Present study |
Little is known about the cytogenetic patterns for Hyphessobrycon, where only a few species have been karyotyped (Table 1), and for many of them, only the haploid number is known (
In the present study, we compare the karyotypes of two populations of Astyanax altiparanae and one of Hyphessobrycon eques aiming to contribute to the increase of knowledge about the patterns of diversity and evolution of karyotype in this incertae sedis group of Characidae.
Materials and methodsSpecimens from two populations of Astyanax altiparanae and one of Hyphessobrycon eques were collected in streams from the upper Paraná river basin (Fig. 1). The individuals were anesthetized with benzocaine (5%) and then sacrificed for subsequent cytogenetic analysis. The processed specimens were fixed in 10% formalin and stored in 70% alcohol for further taxonomic studies. The preserved specimens were placed in the collection of fish from Laboratório de Biologia e Genética de Peixes (LBP), Departamento de Morfologia do Instituto de Biociências da UNESP, campus de Botucatu. Their deposit numbers are indicated below.
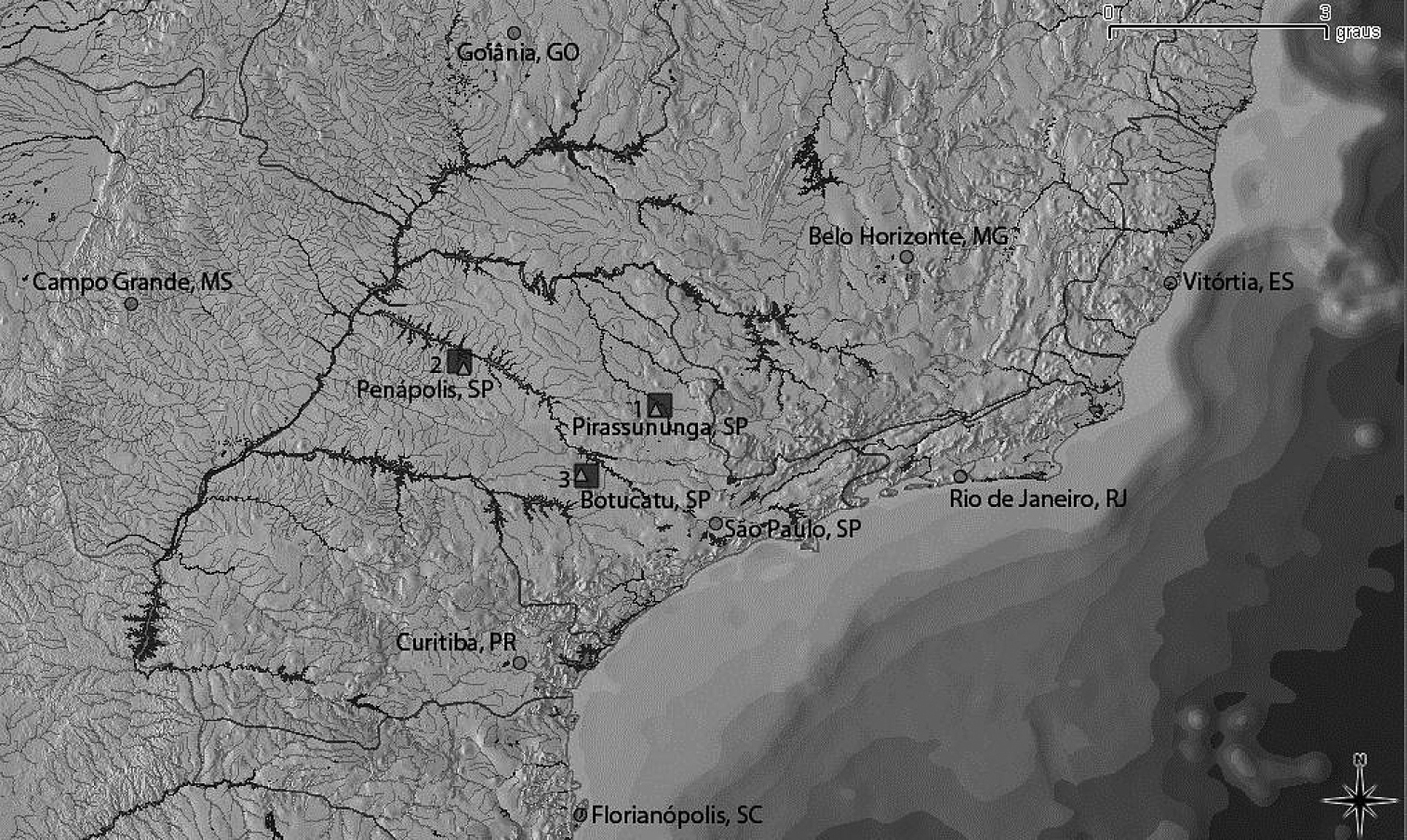
Map of the collection sites (squares) for the Astyanax altiparanae (1, 2) and Hyphessobrycon eques (3) in three rivers of the upper Paraná basin, São Paulo State (SP). Triangles refer to the neighboring cities and circles to the capitals of the states.
The following specimens were karyotyped: six males and four females of Astyanax altiparanae from the Mogi-Guaçu river, Pirassununga, SP., Brazil (Mogi-Guaçu river basin, site 1 in the map, GPS: 21°55'37.6"S, 47°22'04.4"W) with number 1142 (LBP); four males and two females of Astyanax altiparanae from the Tietê river, Penápolis, SP., Brazil (Tietê river basin, site 2, GPS: 21°18'46.1"S, 50°08'26.4"W) with number 2690 (LBP); and three males and two females of Hyphessobrycon eques from the Capivara river, Botucatu, SP., Brazil (Tietê river basin, site 3, GPS: 22°53'57.6"S, 48°23'11.4"W) with number 2337 (LBP) (Fig. 1).
Metaphase chromosomes were studied on slide preparations made from kidney through the common air dryingtechnique (
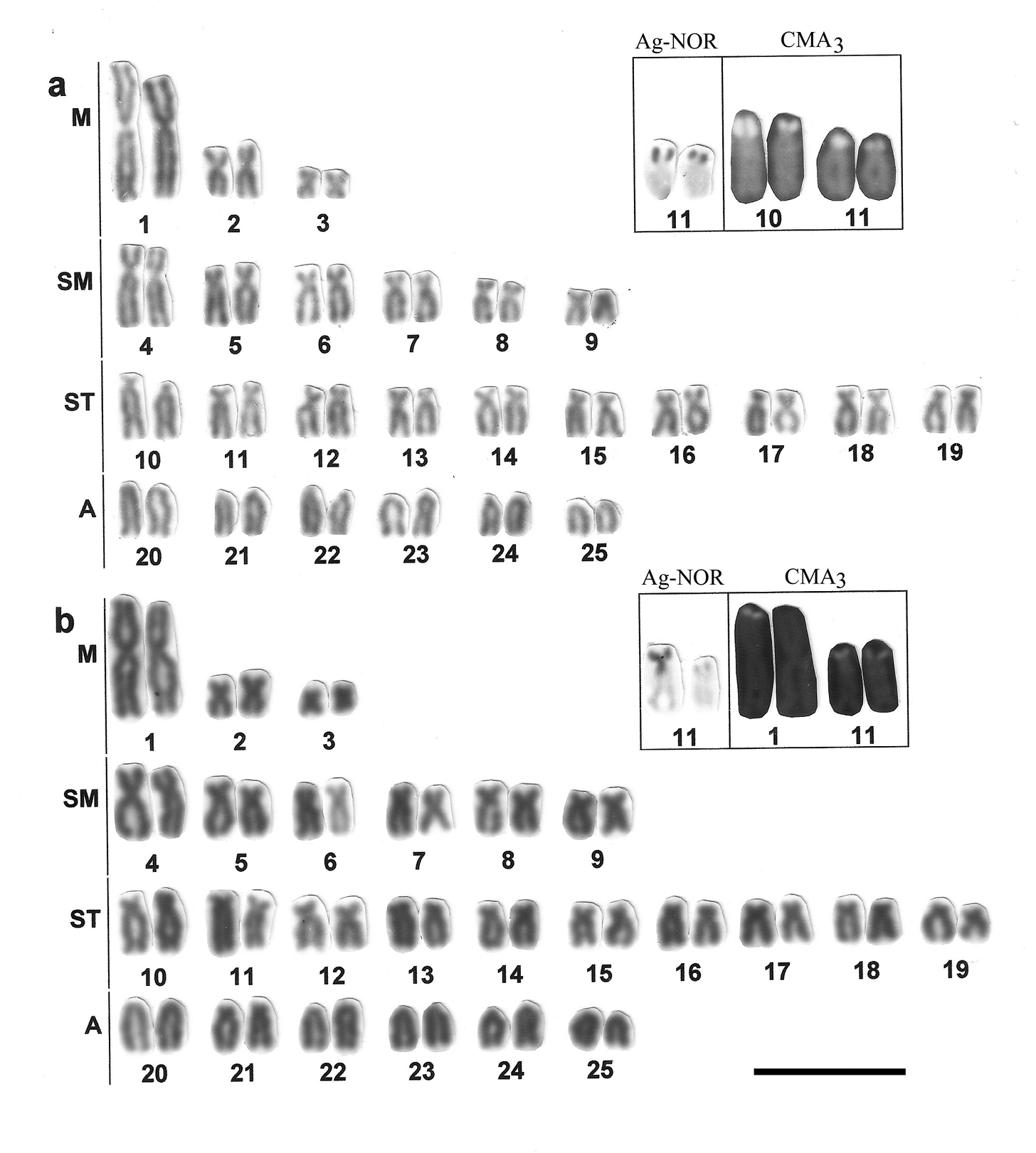
Karyograms showing chromosome morphology with the results of NOR-silver staining and Chromomycin A3 (CMA3) treatment (in a frame) on chromosomes of Astyanax altiparanae from Mogi-Guaçu river (a) and Tietê river (b). Bar = 5µm.
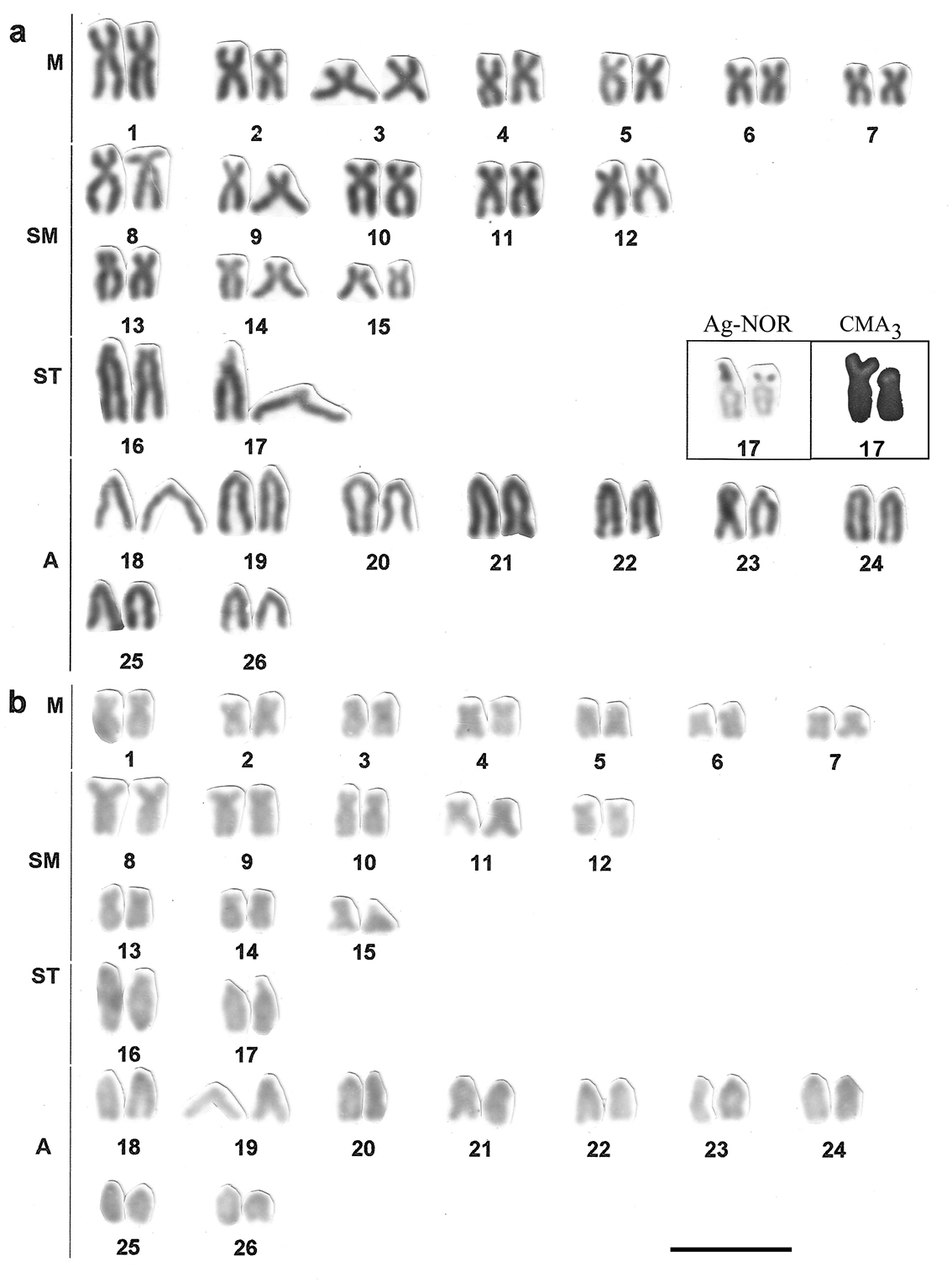
Karyotype of Hyphessobrycon eques showing (a) chromosome morphology with the results of NOR-silver and Chromomycin A3 (CMA3) staining in a frame, and (b) C-banding in fish individuals collected in the Capivara river. Bar = 5µm.
Among the populations of Astyanax altiparanae studied to date, all presented a diploid number of 50 chromosomes, and that is true for the two populations examined in the present study (Table 1). The karyogram of the species contains one big and two small metacentric pairs, a large group of submeta-subtelocentrics and not less than 6 acrocentric pairs (Figs 2a, b). The chromosome morphology did not show populational variations in samples from Mogi-Guaçu river and Tietê river in our data, which share the karyotype formula 6M+12SM+20ST+12A, and fundamental number is accordingly 88.
This chromosomal uniformity is, however, not common for populations from distinct hydrographic basins and even within the same basin, as other populations of the rivers Tiete and Mogi-Guacu basins show considerable variation (Morelli et al. 19983, Neor et al. 2009) (see Table 1).
The intraspecific variety of chromosome formulae in this case is due to the variable content of each morphological group, from M to A, which interpretation may be difficult, however, without chromosome specific markers, still poorly available in ordinary fish cytogenetics. It should be stressed for this species that the presence of the big metacentric (pair M1) seems to be not only the common karyotype feature of species specific significance for Astyanax altiparanae, but also a provisional phylogenetic marker. Taking into account the possibility of appearance of such a large bi-armed chromosome through Robertsonian fusion, it might focus to the karyotype relation between taxa under this study differing in 2n. Arm chromosome variation within and between the karyotypes might be caused by intrachromosomal changes such as pericentric inversion, centromeric shift, heterochromatin or NOR position.
The chromosome number is variable among the Hyphessobrycon species, ranging from 2n=52 chromosomes in Hyphessobrycon herbertaxelrodi (
Impregnation by silver nitrate reveals a single NOR location in a subtelocentric chromosome for populations of Astyanax altiparanae and for the species Hyphessobrycon eques (Figs 2, 3, Table 1). In Astyanax altiparanae, the NOR marks are presented on a short arm of the subtelocentric pair 11 (Figs 2a, b, Table 1). In Hyphessobrycon eques, the similar NOR bearing subtelocentric corresponds to the chromosome 17 in the species karyogram (Fig. 3).
The treatment with fluorochrome Chromomycin A3 (CMA3) was used to evidence NOR as regions associated with GC-rich DNA (
NOR pattern variation has been reported for this species, from single to multiple NORs, which may characterize as intra- as inter-population variation in NOR location and chromosome morphology as well (
In Hyphessobrycon species, too, there is a great variation of NORs appearing as single sites (
According to
The authors thank Renato Devidé for technical assistance. Helpful reviewers comments were appreciated. Financial support of Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Proc. 140644/2005-9 to E.R.M.M.) and Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) is acknowledged.