(C) 2012 Gayane Karagyan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The male karyotypes of Acmaeodera pilosellae persica Mannerheim, 1837 with 2n=20 (18+neoXY), Sphenoptera scovitzii Faldermann, 1835 (2n=38–46), Dicerca aenea validiuscula Semenov, 1895 – 2n=20 (18+Xyp) and Sphaerobothris aghababiani Volkovitsh et Kalashian, 1998 – 2n=16 (14+Xyp) were studied using conventional staining and different chromosome banding techniques: C-banding, AgNOR-banding, as well as fluorochrome Chromomycin A3 (CMA3) and DAPI. It is shown that C-positive segments are weakly visible in all four species which indicates a small amount of constitutive heterochromatin (CH). There were no signals after DAPI staining and some positive signals were discovered using CMA3 staining demonstrating absence of AT-rich DNA and presence of GC-rich clusters of CH. Nucleolus organizing regions (NORs) were revealed using Ag-NOR technique; argentophilic material mostly coincides with positive signals obtained using CMA3 staining.
Coleoptera, Buprestidae, karyotypes, Ag-banding, C-banding, CMA3-staining , DAPI-staining
The family Buprestidae (Coleoptera) is one of the largest groups of Polyphagan beetles containing over than 14500 nominal species worldwide (
Up till now, cytogenetic studies on jewel-beetles have been carried out using conventional staining techniques mainly, with few exceptions.
The present paper is dedicated to study of karyotypes of Acmaeodera pilosellae persica Mannerheim, 1837, Sphenoptera scovitzii Faldermann, 1835, Dicerca aenea validiuscula Semenov, 1895 and Sphaerobothris aghababiani Volkovitsh & Kalashian, 1998 using conventional and differential staining techniques. AgNO3-banding was used to reveal the nucleolus organizing chromosomes and to locate the NORs in them. C-banding method was used to study the distribution of constitutive heterochromatin (CH). To characterize the molecular composition of the CH chromosomes were stained with DNA-specific fluorochromes DAPI and CMA3 which selectively stain AT-rich and GC-rich DNA fragments, respectively. In the present study the fluorochromes CMA3 and DAPI were applied for the first time to jewel-beetles chromosomes.
Material and methodsThe buprestid beetles were collected in 2006 in Southern Armenia (Vayotsdzor and Syunik provinces). Males’ gonads were dissected in several drops of hypotonic 0.9% sodium citrate solution containing 0.005% colchicine and incubated for 30-45 min at room temperature. Then the gonads were fixed in 3:1 ethanol-acetic acid mixture. Karyological slides were made according to the method proposed by
The method is based on selective silver staining of NORs containing clusters of functional rRNA genes. The slides were subjected to hydrolysis in 2N formic acid for 10 min, rinsed in running water and dried. Then the slides were treated by putting of 4-5 drops of 50% aqueous silver nitrate (AgNO3) solution and 2 drops of colloidal developer solution (0.2 g gelatin, 10 ml distilled water and 0.1 ml concentrated formic acid – HCOOH). The slides were covered with a coverslip and incubated on a hotplate for 3-4 min at 600C in a moist chamber (warmed beforehand). After rinsing in distilled water, the slides were dried and stained by 4% Giemsa solution in phosphate buffer (pH 6.8) for 10-20 sec.
The C-banding method (Rożek 2000)The slides were treated for 1-3 min. in 0.2N HCL at room temperature then rinsed in distilled water. Thereafter the slides were placed in 5% Ba(OH)2 solution at 200C for approximately 3 min, then carefully rinsed in streaming and distilled water, and for 1 min in 2xSSC solution (0.3 M sodium chloride containing 0.03 M tri-sodium citrate). Then the slides were incubated in 2xSSC solution at 500C for 1 hr and rinsed in distilled water. Some of these slides were stained with 4% Giemsa solution in phosphate buffer (pH 6.8) and the others were dyed with DNA-specific fluorochromes DAPI and CMA3.
To characterize the molecular composition of the constitutive heterochromatin, we stained the chromosomes with DNA-specific fluorochromes CMA3 (the antibiotic chromomycin A3) and DAPI (4’-6-diamidino-2-phenylindol) that selectively stain GC-rich and AT-rich DNA fragments, respectively (
The slides were analyzed and photographed with a Nikon Eclipse 400 light microscope and CCD DS-U1 camera using the software Lucia Image 5.0.
Data resourcesThe data underpinning the analyses reported in this paper are deposited in the Dryad Data Repository at doi: 10.5061/dryad.hc52qb12.
Results Subfamily Polycestinae Tribe AcmaeoderiniAcmaeodera pilosellae persica, 2n=20, n ♂ = 9 + neo-XY.
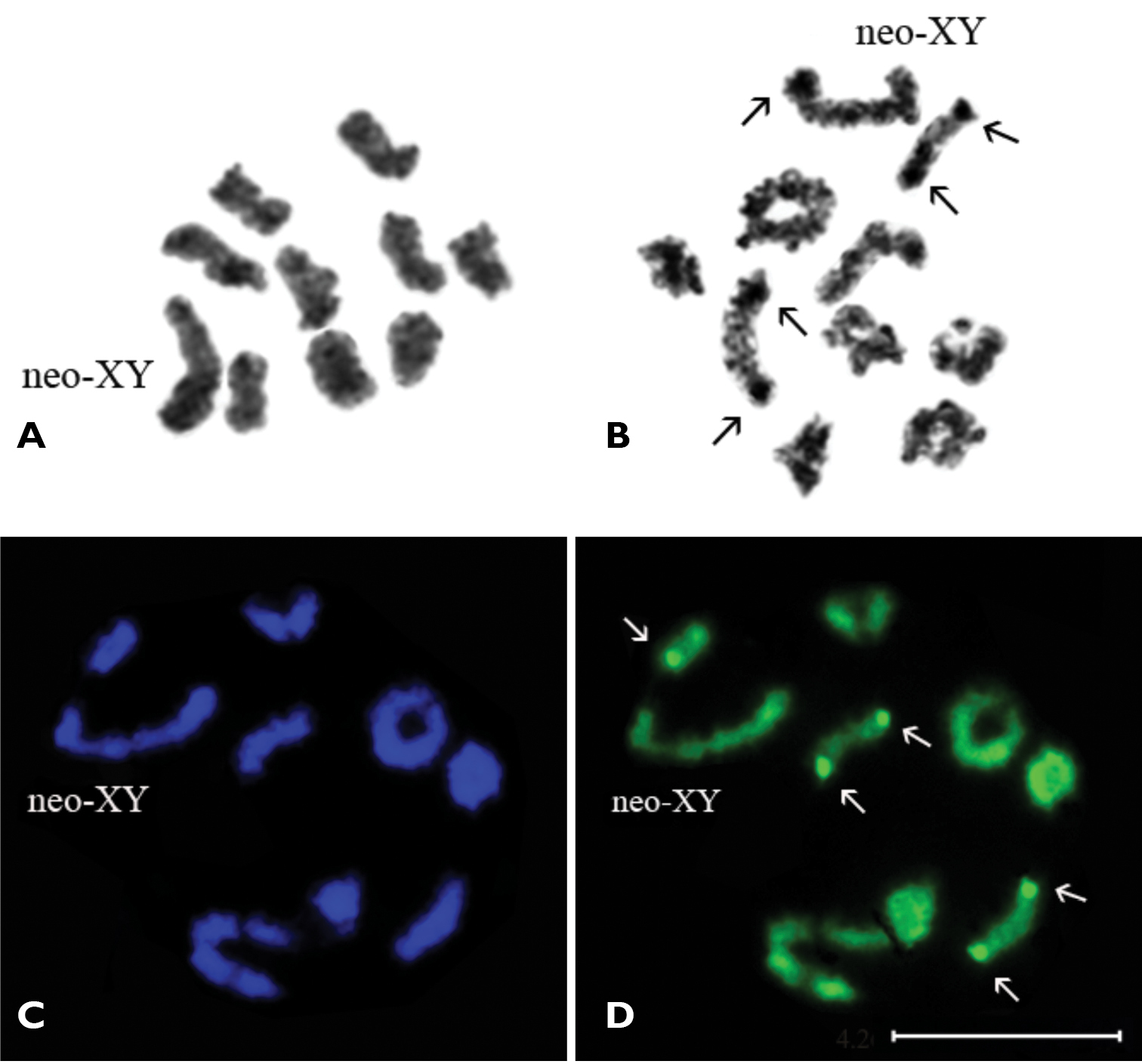
In prometaphase I/metaphase I nine autosomal bivalents formed a series of gradually decreasing sizes and large heteromorphic neo-XY sex-bivalent were observed (Fig. 1A). In late diakinesis / prometaphase I the 5–6 rod-shaped bivalents have most likely a terminal chiasma and 3–4 ring-shaped bivalents – two chiasmata (Fig. 1B). The X-chromosome seems to be submetacentric, Y-chromosome is most probably acrocentric and similar in size to the shorter arm of X-chromosome.
Acmaeodera pilosellae persica, n=9AA+ neo-XY. A prometaphase I/metaphase I B late diakinesis (Ag-banding) C prometaphase I (DAPI staining) D (CMA3 staining). Bar = 10 µm.
The C-banding technique revealed a very small amount of constitutive heterochromatin in the majority of the chromosomes (most probably, in pericentromeric regions) which do not form any distinct blocks. The DAPI staining did not reveal any positive signal and therefore absence of AT-rich clusters of DNA was demonstrated (Fig. 1C). Application of CMA3 staining revealed small positive signals on telomeric regions of both homologues of two or sometimes three rod-shaped autosomal bivalents. Besides, there is small ring-shaped bivalent which is brightly visible in the majority of photographs (Fig. 1D). These signals correspond to argentophilic material revealed by the AgNOR-banding technique (Fig. 1B) and probably associated with NORs. Besides, the Y-chromosome of neo-XY bivalent is dyed argentophilic.
Subfamily Chrysochroinae Tribe SphenopteriniSphenoptera scovitzii, 2n=38–46.
The karyotype of Sphenoptera scovitzii studied using Ag-banding was published earlier (
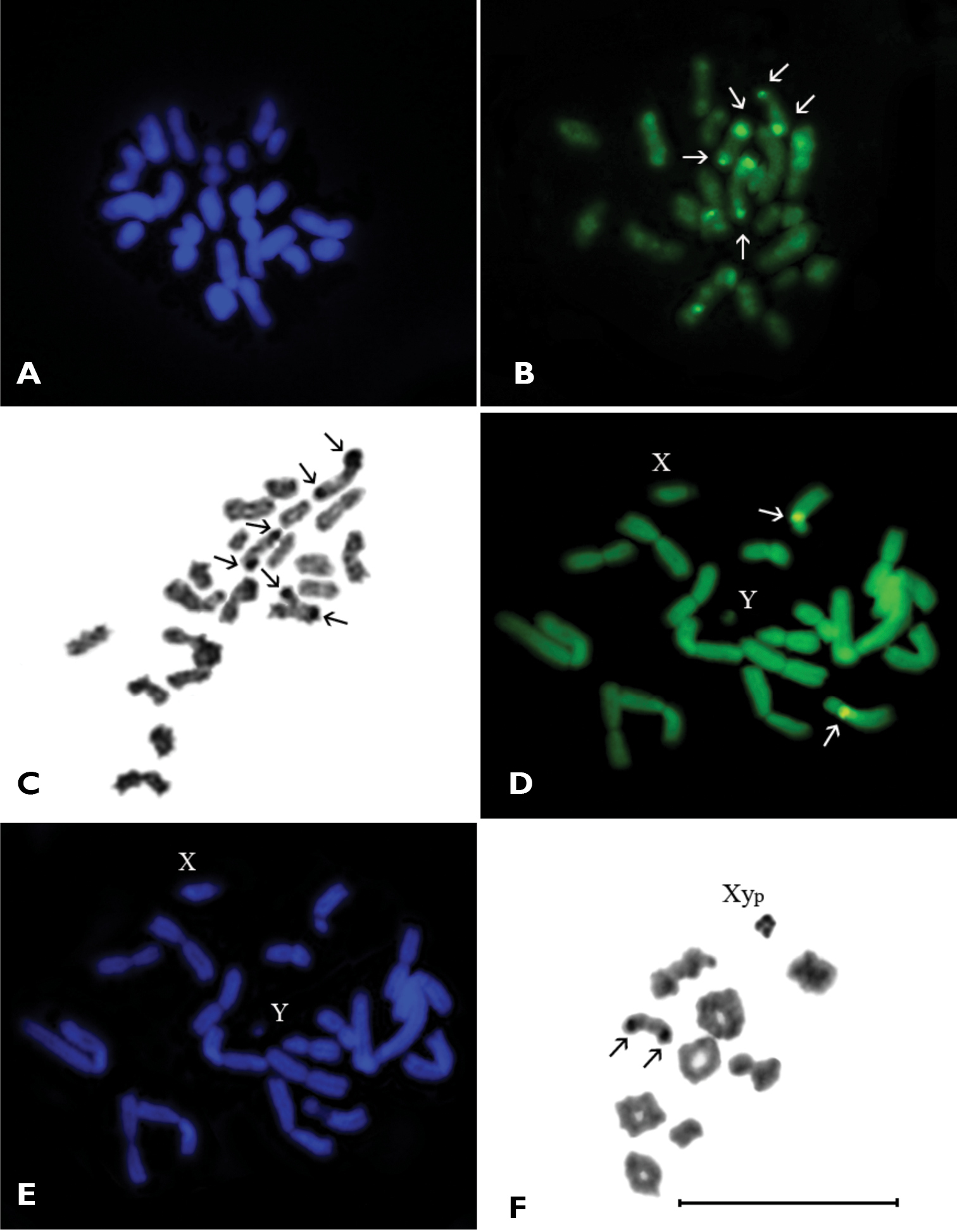
Some of chromosomes have a very small amount of constitutive heterochromatin weakly visible in pericentromeric regions and do not form distinct blocks. The DAPI staining of chromosomes did not reveal any positive signal (Fig. 2A), yet fluorescence after CMA3 staining was discovered (Fig. 2B). In metaphase I, three or sometimes four rod-shaped bivalents showed distinct CMA3 positive signals on telomeric regions of both homologues. These signals are quite stable in the largest and in two of the middle-sized bivalents and correspond to Ag-positive material revealed by the AgNOR-banding technique (Fig. 2C). Weak CMA3 positive signals were also at times visible in some other small bivalents.
A–C Sphenoptera scovitzii, n=19-23, metaphase I. A DAPI staining; B CMA3 staining; C Ag-banding D–F Dicerca aenea validiuscula, n=9AA+XypD mitotic metaphase (CMA3 staining) E mitotic metaphase (DAPI staining) F diakinesis/prometaphase I (Ag-banding). Bar = 10 µm.
Dicerca aenea validiuscula, 2n=20 (18+Xyp).
The male mitotic metaphase displayed 20 chromosomes including 9 autosomal pairs and X- and Y- sex chromosomes (Fig. 2D). All autosomes were biarmed: one pair large and 5 pairs of middle-sized metacentrics, 2 pairs of middle-sized submetacentrics and 1 pair of middle-sized subtelocentric. The X-chromosome was middle-sized and acrocentric, Y-chromosome was dot-like with unclear morphology. In mitotic metaphase CMA3 positive signals were found in the pericentromeric region of long arm of middle-sized homologous pair of subtelocentric autosomes (Fig. 2D). The DAPI staining did not reveal any positive signal (Fig. 2E).
In diakinesis/prometaphase I nine autosomal bivalents and heteromorphic sex-bivalent most probably of “parachute” Xyp type were observed (Figs 2F, 3A). The autosomal bivalents formed a series of gradually decreasing sizes. There were 5–6 ring-shaped autosomal bivalents with two chiasmata, 1–2 rod-shaped bivalents possessed most likely one terminal chiasma and 1–2 were cross-shaped with an interstitial chiasma. The heterovalent Xyp was rather small. In the middle-sized rod-shaped bivalent (which, most likely, formed by subtelocentric autosomes) the CMA3 positive signals were visible in terminal regions of both homologues (Fig. 3A). At this stage staining by AgNOR-banding revealed Ag-positive signals on the same bivalent, besides the sex bivalent was nearly homogenously argentophilic (Fig. 2F).
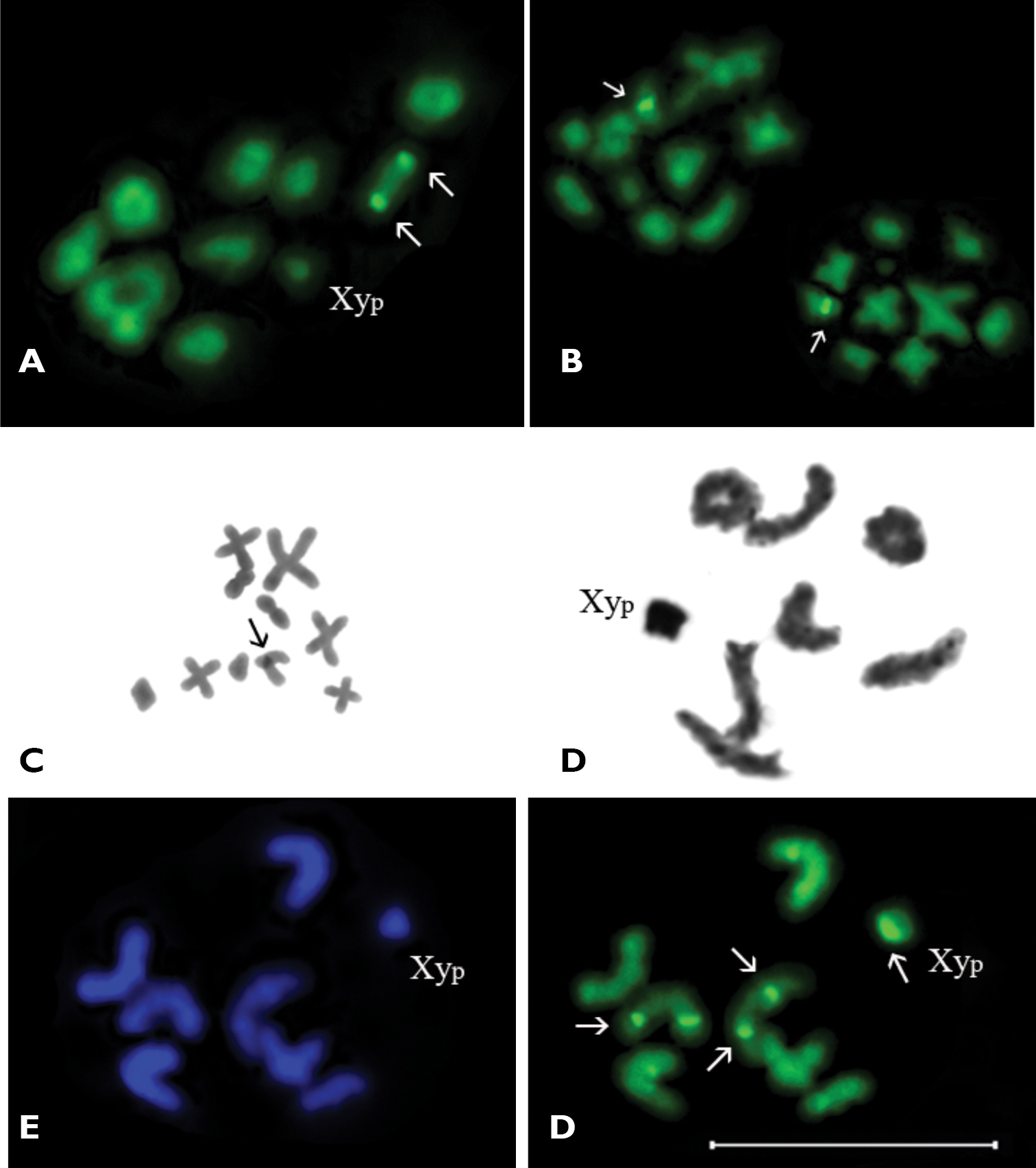
A–C Dicerca aenea validiuscula, A diakinesis/prometaphase I (CMA3 staining) B metaphase II (CMA3 staining) C metaphase II (C-banding) D–F Sphaerobothris aghababiani, n=7AA+XypD diakinesis/prometaphase I (Ag-banding) E metaphase I (DAPI staining) F metaphase I (CMA3 staining). Bar = 10 µm.
Metaphase II showed 10 chromosomes of which 7 chromosomes were meta- and submetacentric, one of chromosome was subtelocentric (which showed CMA3 positive signal on pericentromeric region), whereas the morphology of other chromosomes was vague (Figs 3B, 3C). The C-banding revealed weakly visible constitutive heterochromatin localized in the pericentromeric region of the same subtelocentric chromosome (Fig. 3C).
Subfamily Buprestinae Tribe ChrysobothriniSphaerobothris aghababiani, 2n=16 (14+Xyp).
In diakinesis/prometaphase I seven autosomal bivalents and a sex chromosome heterovalent of the Xyp type were observed (Fig. 3D). The bivalents gradually decreased in size. The Xyp sex heterovalent was smallest element in the set. The majority of autosomal bivalents were rod-shaped, but in some cells one or two ring-shaped autosomal bivalents were observed.
The C-banding revealed a small amount of constitutive heterochromatin most probably in the pericentromeric regions and do not form distinct blocks. The DAPI staining did not reveal any positive signals (Fig. 3E). In metaphase I bright and distinct CMA3 positive signal was visible on “parachute” sex heterovalent, probably connected with the Y-chromosome (Fig. 3F). In addition, weak CMA3 positive signals were observed, as a rule they were situated in telomeric or near telomeric regions of some of rod-shaped autosomal bivalents. Ag-banding revealed the bright argentophilic material connected with whole “parachute” type sex heterovalent (Fig. 3D). Small and weak argentophilic blocks were visible in telomeric or near telomeric regions of some autosomal bivalents.
DiscussionIn total, the karyotypes of 92 species of jewel-beetles belonging to 23 genera, 14 tribes of 5 subfamilies are presently described. Generalization of data including new ones shows that the modal diploid chromosome number in Buprestidae is 2n=20 (9AA + X- and Y- sex chromosome heterovalent in males) so far found in 18 species belonging to 8 genera, 6 tribes, 4 subfamilies. The Xyp type of sex chromosome heterovalent is modal and occurs in 66 species, 16 genera, 10 tribes, 4 subfamilies. The most common karyotype 2n=20 (19+Xyp) can be considered as modal. The new data confirm modality of this karyotype within the family. This karyotype occurs in a large number of beetles from different families (
Application of C-banding technique showed that the studied species of jewel-beetles are characterized by a small amount of heterochromatin localized, most probably, in pericentromeric regions of chromosomes, as in most studied species of the order Coleoptera (
Until now, the karyotypes of only seven species of jewel-beetles studied by using AgNOR-staining technique have been published (
Even this restricted material showed noticeable variability of distribution of argentophilic material (probably NOR) in the karyotypes of jewel-beetles. The argentophilic material is located on:
1) the autosomes: Sphenoptera scovitzii (2n=38–46), Sphenoptera mesopotamica Marseul, 1865 (2n=24, Xyp).
2) both on the sex chromosomes and on the autosomes: Acmaeoderella villosula Steven, 1830 (described as Acmaeoderella boryi Brullé, 1832 in
3) sex chromosomes only, situating either on one of the sex chromosomes or localized between sex chromosomes of bivalent: Acmaeoderella flavofasciata (Piller & Mitterpacher, 1783) (2n=18, Xyr), Acmaeoderella gibbulosa Ménétriés, 1832 (2n=18, Xyr), Acmaeoderella vetusta (Ménétriés, 1832) (2n=18, Xyr). In Euchroma gigantea (2n=32, 36, X1X2X3Y1Y2Y3 –
As commonly understood, in Coleoptera NORs may be located in some autosomal pair and/or sex chromosomes (
Chromosome staining by DNA base specific fluorochromes has been used in cytogenetic studies of Coleoptera (
The correlation between NORs and CMA3 bands is quite common in some insects, including beetles (
Fluorochrome DAPIstaining in all studied species of jewel-beetles did not reveal any particular bright regions on chromosomes which allows for preliminary suggestion that CH have no distinct AT-rich DNA clusters. In general, there is variable distribution of AT- or GC-rich clusters of CH among beetles studied by fluorochromes staining. For instance, positive CMA3 and negative DAPI signals were found in some Elateridae (
In conclusion, data of the present study offer important insights into the karyotypes characteristics of jewel-beetles which may be useful in elucidation of relationships both among the species of the family itself as well as between jewel-beetles and the representatives of other coleopteran families.
This study was supported by Józef Mianowski Fund (Poland). We would like to express our greatest gratitude to our colleagues from the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences Prof. dr hab. Elżbieta Warchałowska-Śliwa, Prof. dr hab. Anna Maryańska-Nadachowska, Dr. Maria Rożek and Prof. dr hab. Adam Nadachowski for their invaluable support of this study. We gratefully acknowledge to Dr. Alexander Danchenko (Moscow State University, Moscow, Russia) for his kind help in collecting some of the specimens studied.