(C) 2012 Alexandra M. R. Bezerra. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Clyomys Thomas, 1916 is a semifossorial rodent genus of spiny rats represented by only one species, Clyomys laticeps, which inhabits the tropical savannas and grasslands of central Brazil and eastern Paraguay. Here we describe a new karyotype of Clyomys laticeps found in populations of Emas National Park, Goiás state, Brazil. The four analyzed specimens had a diploid number (2n) of 32 and a fundamental autosome number (FN) of 54. Cytogenetic data include conventional staining, CBG and GTG-banding. The karyotype presents 12 meta/submetacentric pairs (1 to 12) and 3 pairs of acrocentrics (13 to 15) with gradual decrease in size. The X chromosome is a medium submetacentric and the Y is a medium acrocentric. The semifossorial habits together with habitat specificity could have contributed to the karyological variations found on this genus.
Clyomys, Cerrado, cytogenetic, Echimyidae, taxonomy, semifossorial habit
The genus Clyomys Thomas, 1916 has long been represented by two living species, namely Clyomys laticeps and Clyomys bishopi Avila-Pires et Wutke, 1981distributed in tropical savannas and grasslands from circa 100 to 1, 100 m elevation in central Brazil and eastern Paraguay (
Spiny rats of the genus Clyomys present semifossorial habit and can be identified, together with the other semifossorial echimyids genera Carterodon Waterhouse, 1848and Euryzygomatomys Goeldi, 1901 by a set of morphological characters such as a body covered by spinous pelage, short tail and limbs, and long, powerful claws (
Cytogenetic studies of the genus Clyomys reported a diploid number (2n) of 34 chromosomes and fundamental autosome number (FN) 60 or 62. The population from State of São Paulo, Brazil, described by
The present paper describes a different diploid number for Clyomys laticeps from a Central Brazilian sample. We also discuss habitat use and biology of this species and their bearing on the observed intraspecific karyotypic variation.
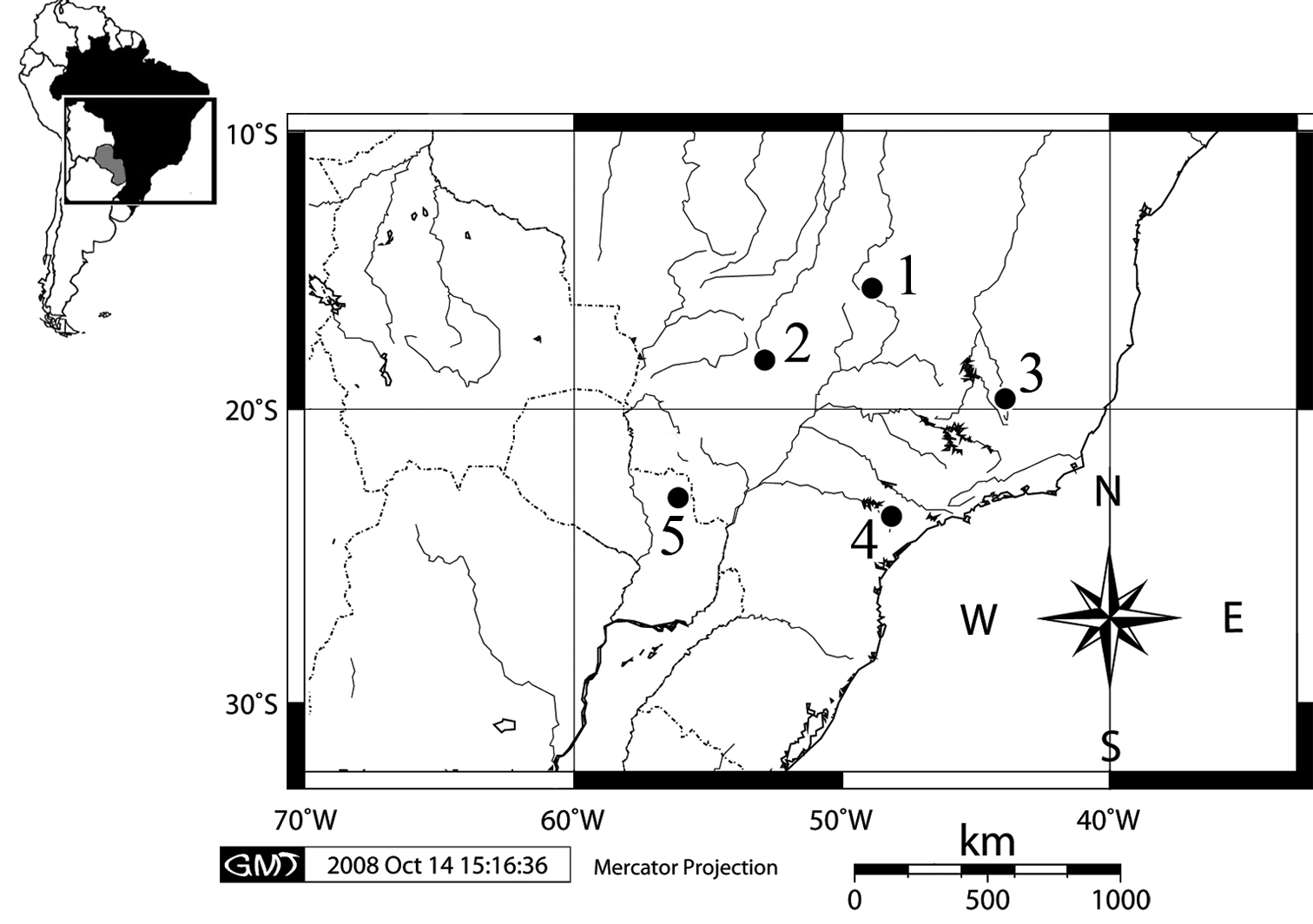
Material and methodsFour wild-caught specimens (2 females and 2 males) of Clyomys laticeps from Emas National Park (ENP), state of Goiás, Brazil (18°15'50"S, 52°53'33"W) were karyotypically studied (Fig. 1). The vouchers specimens are deposited at the Museu Nacional (MN), Universidade Federal do Rio de Janeiro, and at the Mammal Collection of the Universidade de Brasília (UNB), Brazil: MN 68165 (female), MN 68164 (male), MN 68167 (male), and UNB 2155 (female). The map was generated using the software
Map of karyotyped populations showing the type localities of Clyomys laticeps and its synonyms: Brazil, Distrito Federal, Brasília [1] (
Mitotic metaphase cells were obtained from bone marrow and spleen after in vivo colchicine treatment. Mitotic cells were spread onto clean glass slides, air-dried and stored at -20o C until use. Analysis were performed after routine Giemsa staining, CBG-banding (
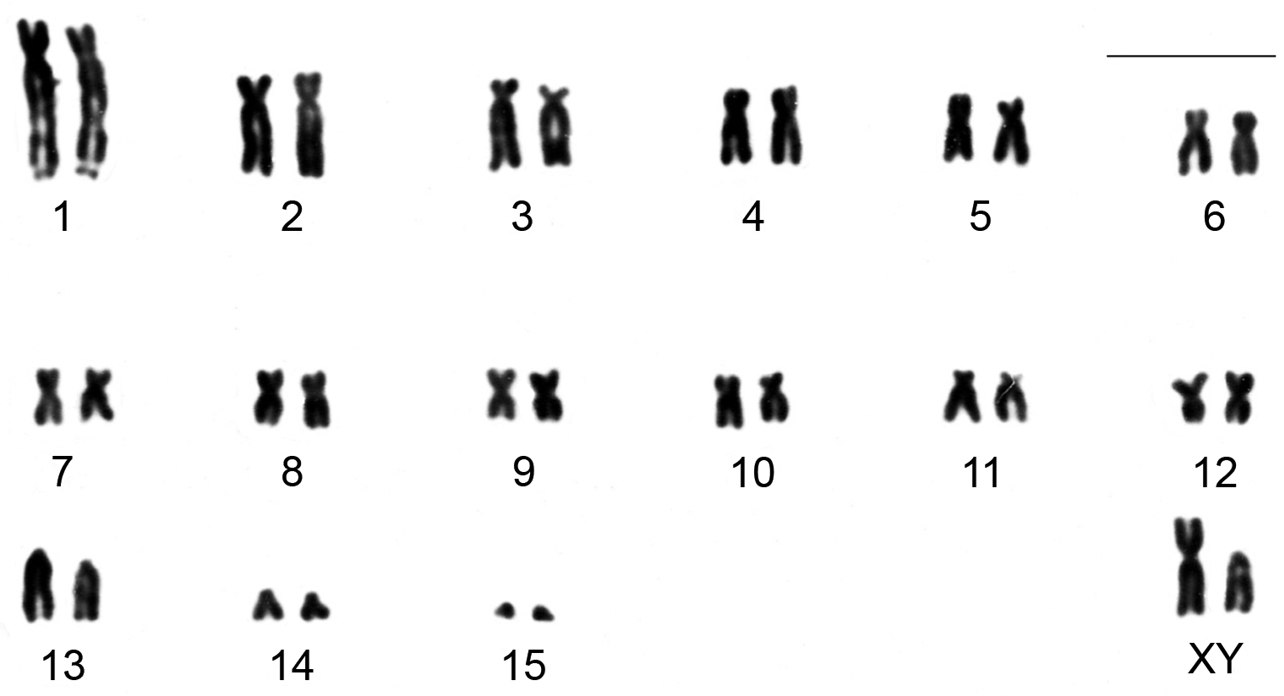
Clyomys laticeps from ENP shows a karyotype with 2n = 32 and FN = 54. The autosome complement comprises 12 biarmed pairs (pair 1 is submetacentric with a distal secondary constriction in the long arm, pairs 2 to 12 are metacentric or submetacentric chromosomes) and three acrocentric pairs (pair 13 a heteromorphic medium acrocentric and pairs 14 and 15 are small acrocentrics). The X chromosome is submetacentric and Y is acrocentric, both morphologically distinguishable after G and/or C banding pattern (see below and Fig. 2).
Karyotype of a male of Clyomys laticeps (MN 68164) from Emas National Park after conventional staining (2n = 32, FN = 54). Bar = 10 μm.
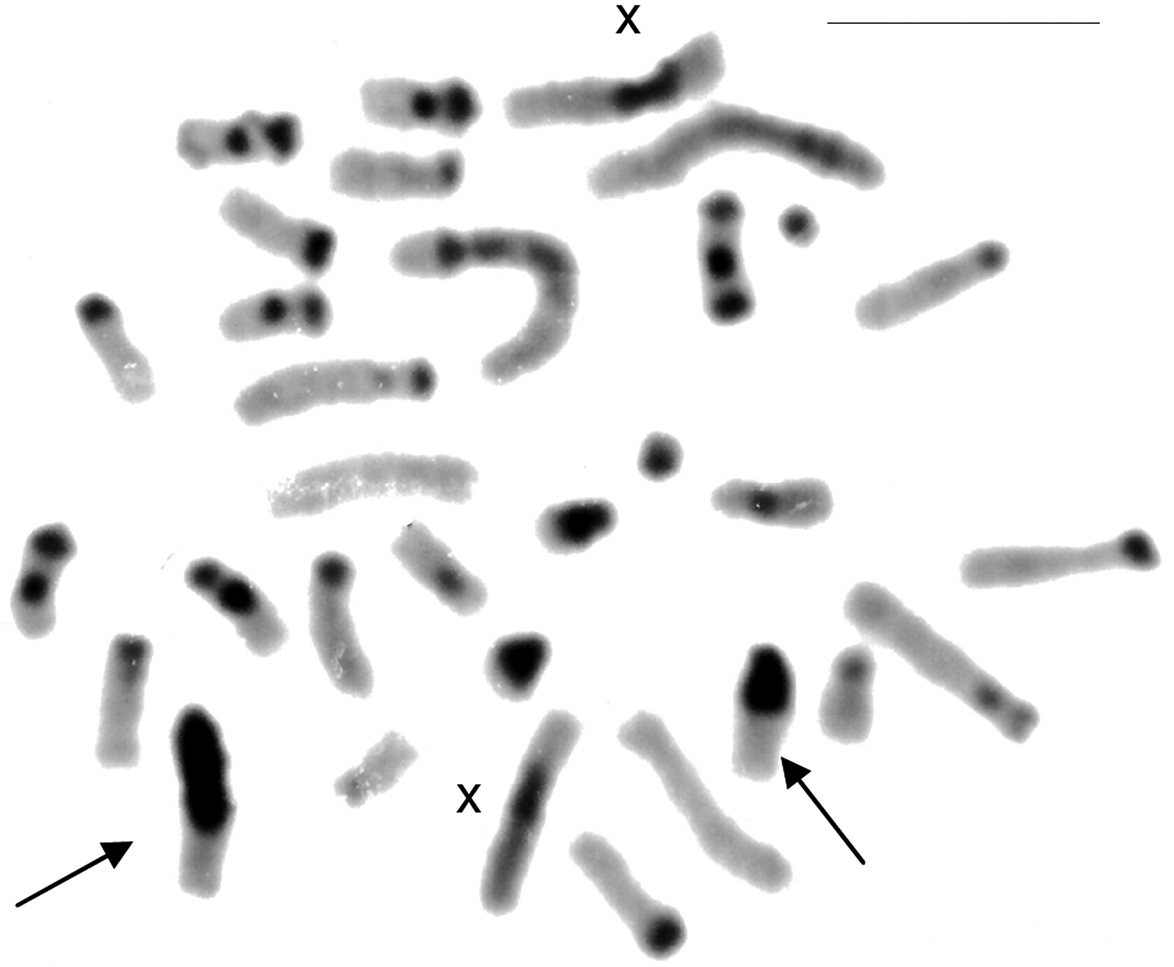
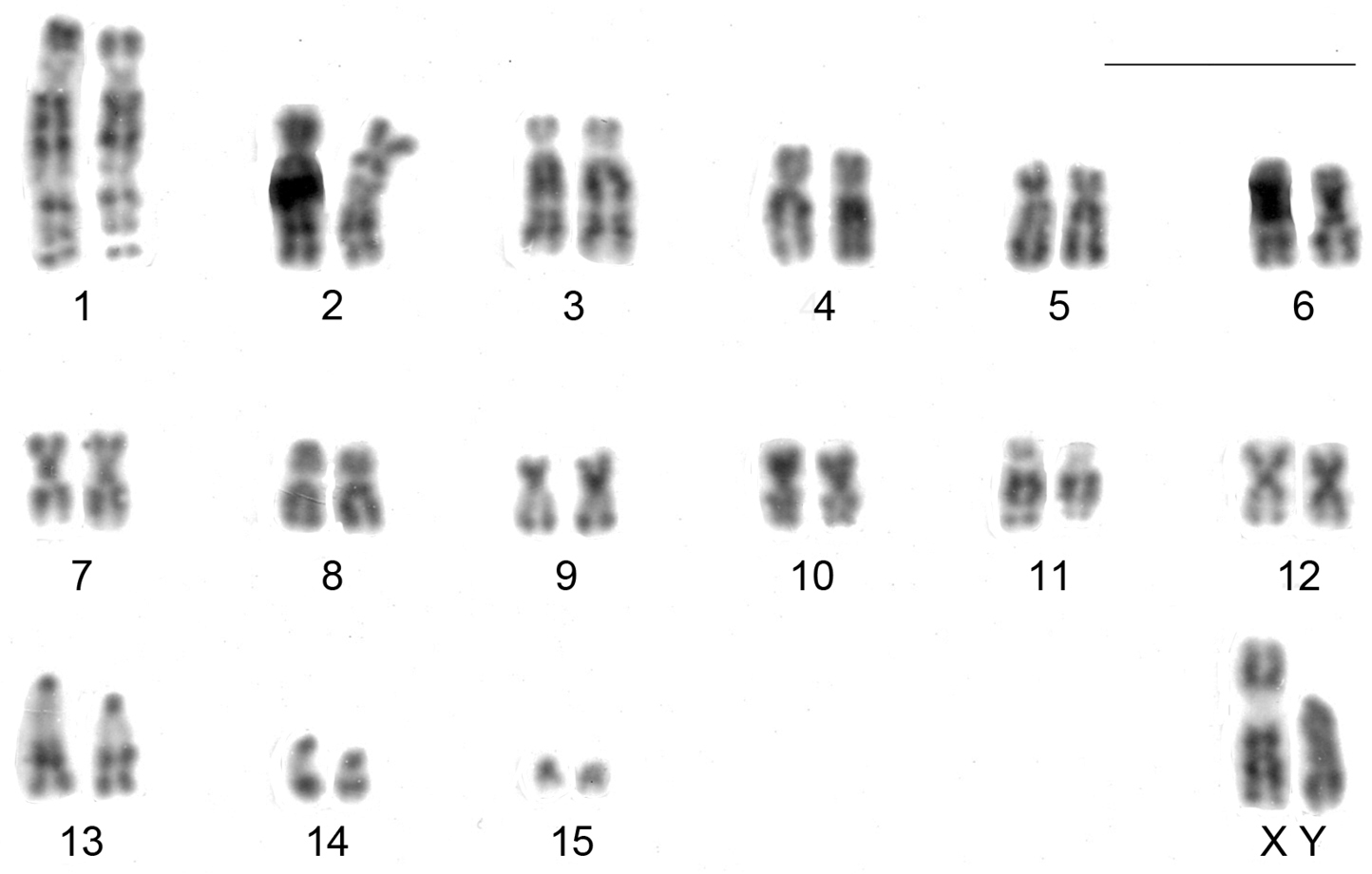
CBG-banding revealed constitutive heterochromatin at the telomeric regions of some autosomes (pairs 2 and 4–10). Additionally, interstitial bands occurred in the pericentromeric region of pairs 2 and 8–10. The distal secondary constriction in the long arm of pair 1 is C-band negative and the proximal region of both arms shows a small amount of faintly stained constitutive heterochromatin. Pair 3 is completely C-band negative. Pericentromeric positive C-band was present in the pairs 11 and 12, as well as in pairs 13 and 14, which additionally show a large block of heterochromatin in the proximal region of the long arm. The 15th pair is completely heterochromatic. The 13th autosome pair is heteromorphic due to the size of constitutive heterochromatin in all studied specimens (Fig. 2). The X chromosome is identifiable by a large submetacentric with distinctive centromeric heterochromatin and by an unique G-banding pattern characterized by a wide negative G-band at pericentromeric region (Figs 3 and 5). The Y chromosome is an acrocentric similar in size to the smaller acrocentric of the pair 13. It has a conspicuous C-positive band segment in the pericentromeric region and a block at the proximal region of the long arm (Figs 3 and 4). It is readily identifiable only after G-banding since it is G-positive along all its length comparing to the autosome pair 13 (Fig. 5).
CBG-banded karyotypes of female of Clyomys laticeps (UNB 2155) from Emas National Park (2n = 32, FN = 54). Inset: sex chromosomes of a male (MN 68165). Bar = 10 μm.
CBG-banded metaphase of a female of Clyomys laticeps (UNB 2155) from Emas National Park (2n = 32, FN = 54). The arrows indicate the heteromorphic 13th pair. Bar = 10 μm.
GTG-banded karyotypes of a male of Clyomys laticeps (MN 68164) from Emas National Park (2n = 32, FN = 54). Bar = 10 μm.
The cytogenetic analysis carried out in Clyomys laticeps from Emas National Park, Goiás state, Brazil, revealed a new karyotype, with 2n = 32, FN = 54. The specimens of Clyomys laticeps described in the literature from São Paulo state and from Distrito Federal, respectively, shared very similar 2n = 34 karyotypes with a minor difference only in fundamental autosome number (60/62). Specimens from Itapetininga, São Paulo state, the type locality of Clyomys bishopi, also showed 2n = 34 and FN = 60 (
The cytogenetic distinction between 2n = 34 karyotypes from São Paulo (with FN = 60) and Distrito Federal (FN = 62) could be the result of a rearrangement such as a pericentric inversion on one pair of chromosomes. The difference in diploid number between the karyotypes of Clyomys laticeps with 2n = 32 and 2n = 34, in the other hand, might mostly be related to Robertsonian rearrangements (fusion/fission events).
The origin of the diploid number differences is probably the result of a series of complex rearrangements. The karyotypes with 2n = 34 (from São Paulo specimens) are composed by two small pairs of acrocentric chromosomes (15 and 16) while in specimens with 2n = 32 from ENP there are three pairs of small acrocentrics (13, 14 and 15). There is correspondence between the C-band pattern between the pairs 15 and 16 of Clyomys laticeps from São Paulo stateand the pairs 14 and 15 from the ones of ENP. Therefore, the karyotypes with the smallest diploid number present an additional small acrocentric pair (13). Moreover, the first pair of chromosomes in the karyotype of São Paulo specimens (2n = 34) is a large acrocentric with a small quantity of heterochromatin in the pericentromeric region, while the first pair in ENP specimens (2n = 32) is a submetacentric with a small amount of faintly stained constitutive heterochromatin in the proximal region of both arms. The X chromosome is also morphologically distinct between the karyotypes analyzed, being an average sized acrocentric in São Paulospecimensand a submetacentric in specimens from ENP. The constitutive heterochromatin in the X chromosome of 2n = 34 karyotypes is located in the pericentromeric region and in the proximal region of the long arm, while in the X chromosome of karyotypes with 2n = 32 a pericentromeric heterochromatic band is present. Thus, events such as addition/deletion of heterochromatin and pericentric inversions associated with centric fission/fusion (Robertsonian rearrangements) might have happened in the evolutionary differentiation of the karyotypes of these two populations.
Cytogenetic variability in fossorial and semifossorial rodents has been widely reported in the literature (e.g.,
The distinct diploid number shown by Clyomys populations (
We thank João A. Oliveira (MN), Stella Franco (MN), and Jader Marinho-Filho (UnB) for hospitality in the collections and for cataloguing the voucher specimens, respectively; to Albert D. Ditchfield, Riccardo Castiglia, and Marta Svartman for critically reviewing early versions of the manuscript; Reginaldo Constantino provided the database for the map confection. This study was part of the project “Vertebrates of Emas National Park” supported by Fundação O Boticário de Proteção à Natureza/ MacArthur Foundation, Wildlife Conservation Society, BP Conservation/ BirdLife International/ Fauna and Flora International and Conservation International do Brasil/ Anhauser-Bush, and also part of the MSc dissertation of AMRB developed at MN, who received a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We are grateful to the IBAMA for the research permission and the support at ENP (proc. 02001.001282/97–01, license 056/1999) AMRB received a postdoctoral fellowship an FHGR a research grant from CNPq (CNPq Procs. 150599/2008–0 and 300664/2008–7, respectively).