(C) 2012 Robert B. Angus. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
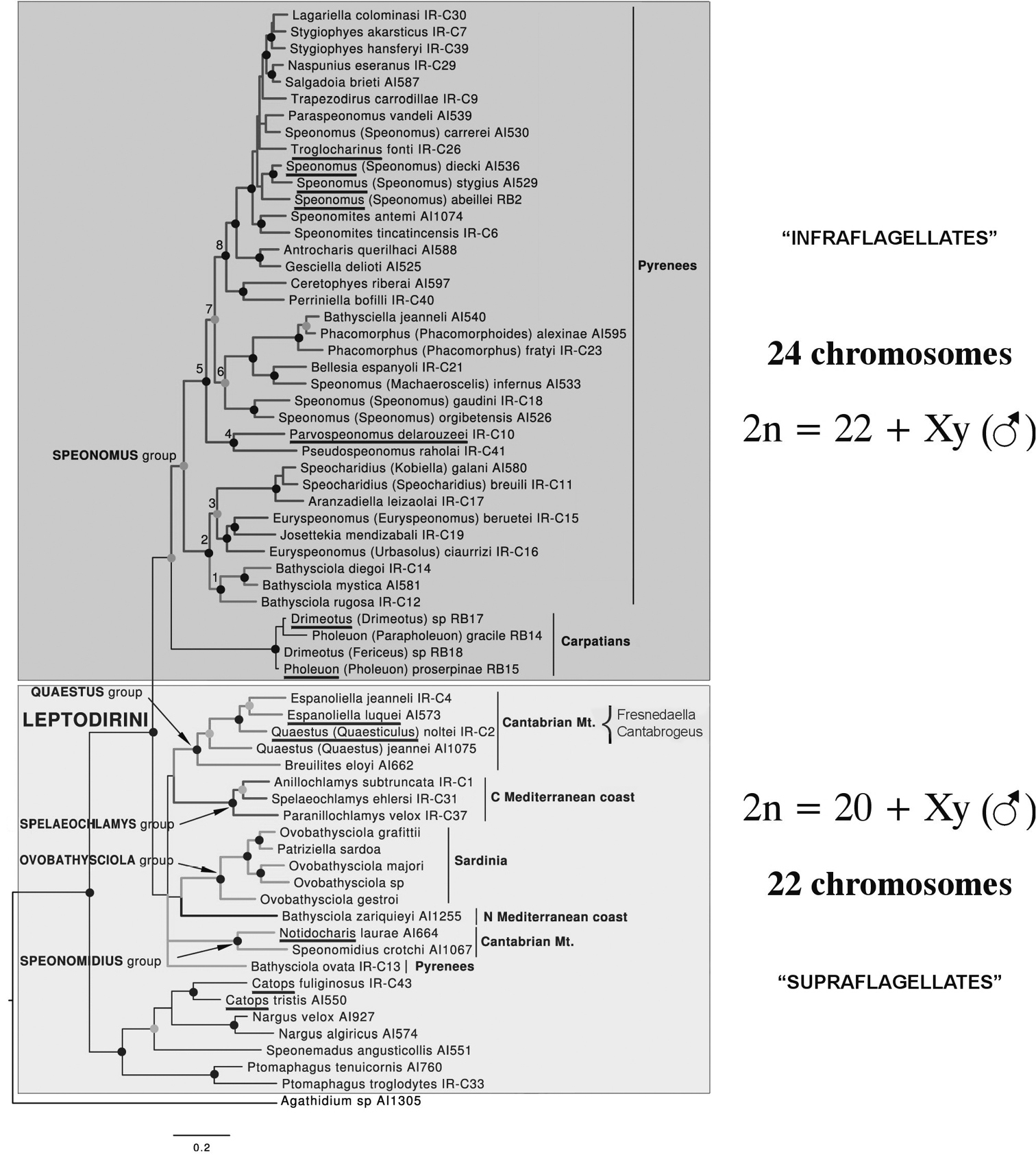
Karyotypes are shown for Leiodes calcarata (Erichson, 1845), Catops coracinus Kellner, 1846, Cantabrogeus luquei (Salgado, 1993), Espanoliella luquei Salgado & Fresneda, 2005, Fresnedaella lucius Salgado, Labrada & Luque, 2011, Notidocharis uhagoni (Sharp 1872), Quaestus (Quaesticulus) pasensis Salgado, Labrada & Luque, 2010, all of which are shown to have a diploid number of 20 autosomes plus Xy (♂) or XX (♀) sex chromosomes, as well as an as yet undescribed triploid species of the genus Cantabrogeus Salgado, 2000. These results are contrasted with published information, all on Leptodirini, which lists 10 species as having diploid numbers of 22 + Xy or XX. It is shown that the higher chromosome number (n = 11 + X or y) previously reported refers exclusively to the more derived Leptodirini (“infraflagellates”) whereas the lower number (n = 10 + X or y) refers to the less derived surface-dwelling forms and the less derived Leptodirini (“supraflagellates”).
Chromosomes, Leiodidae, Leptodirini, Leiodes Latreille, 1796 , Catops Paykull, 1798 , Cantabrogeus Salgado, 2000 , Espanoliella Georgiev, 1976 , Fresnedaella Salgado Labrada & Luque, 2011 , Notidocharis Jeannel, 1956 , Quaestus Schaufuss, 1861 , Cantabria, triploid
Published information on the chromosomes of Leiodidae is very limited and refers exclusively to subterranean species of the tribe Leptodirini (
However, investigation of two surface-dwelling forms in 2006 by D.B. Edwards as part of a student project supervised by R.B. Angus, revealed the diploid number 20 + Xy in the males studied. This was a surprise and remained a puzzle until, in December 2009 R.B. Angus received a request from I. Ribera (Barcelona) for help in determining the chromosome number of a cave-dwelling leptodirine from the Cantabria region of northern Spain. Despite intensive searching by C.G. Luque and L. Labrada, only females of this species had been found in the cave so that they suspected the species might be parthenogenetic and wondered if it might be triploid. Investigation of this material showed that the species was indeed triploid, with consistent finding of 33 chromosomes per nucleus. However, while the confirmation that this was a triploid parthenogenetic species was very satisfying, it also added to the puzzle over the diploid number for Leiodidae as this species is was a subterranean member of the tribe Leptodirini, from which the diploid number 24 had been consistently reported. It was therefore decided to extend the investigation to some of the bisexual species to see what the diploid number was in species from Cantabria.
The tribe Leptodirini Lacordaire, 1854 (= Bathysciini Reitter 1906) of the family Leiodidae (
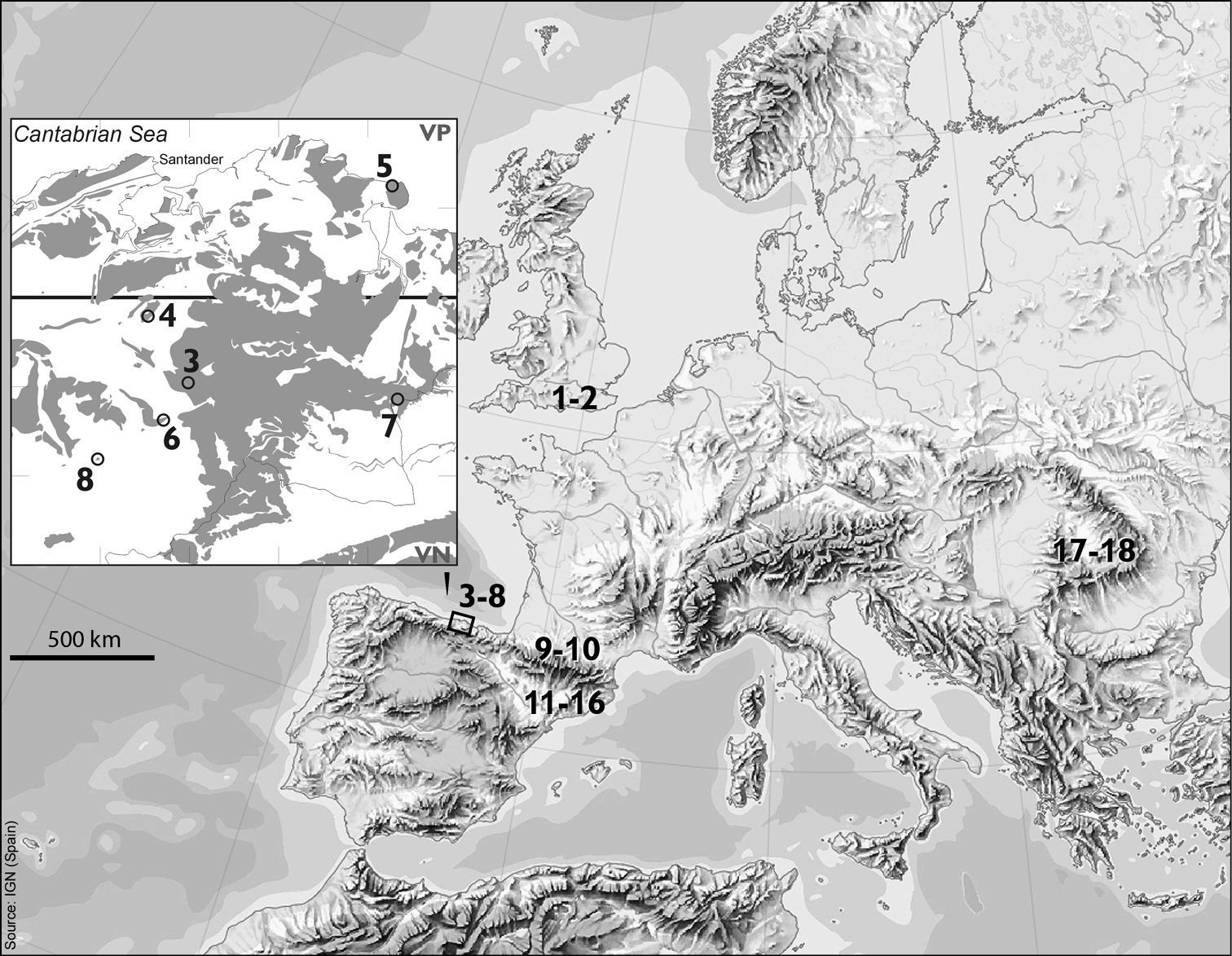
The material investigated is listed in Table 1, and the localities from which the material was obtained are marked on the maps shown in Fig. 1. In all cases the number of specimens from which successful preparations have been obtained is given. As these beetles are frequently very small (2 mm or less) the success rate was generally low and preparations were attempted on considerably more specimens than are listed as successful. It should also be noted that there was often considerable mortality in transit, even when material was sent express in cooled containers.
Material used, localities, map numbers, numbers of specimens giving successful preparations.
| Species | Locality with No. on map | Number giving successful preparations |
|---|---|---|
| Leiodes calcarata (Erichson, 1845) | ENGLAND. Surrey: Virginia Water (No. 1) | 1♂ |
| Catops coracinus Kellner, 1846 | ENGLAND. Surrey: Virginia Water (No. 2) | 1♂ |
| Cantabrogeus sp. (triploid) | SPAIN. Cantabria: Municipal District of San Roque de Riomiera, Covallarco cave (No. 3) | 7♀♀ |
| Cantabrogeus luquei (Salgado, 1993) | SPAIN. Cantabria: Municipal District of Penagos, Los Gentiles cave (No. 4) | 2♂♂, 1♀ |
| Espanoliella luquei Salgado & Fresneda, 2005 | SPAIN. Cantabria: Municipal District of Santoña, Merino cave (No. 5) | 1♂, 1♀ |
| Fresnedaella lucius Salgado, Labrada & Luque, 2011 | SPAIN. Cantabria: Municipal District of Selaya, La Canal de la Cubía cave (No. 6) | 1♂, 1♀ |
| Notidocharis uhagoni (Sharp, 1872) | SPAIN. Cantabria: Municipal District of Ramales, Cullalvera cave-entrance (No. 7) | 2♂♂ |
| Quaestus (Quaesticulus) pasensis Salgado, Labrada & Luque, 2010 | SPAIN. Cantabria: Municipal District of Luena, El Rellano del Mazo cave (No. 8) | 2♂♂ |
The methods of chromosome preparation are as described by
For assessment of the chromosomal data in terms of DNA-derived phylogeny we used the dataset from
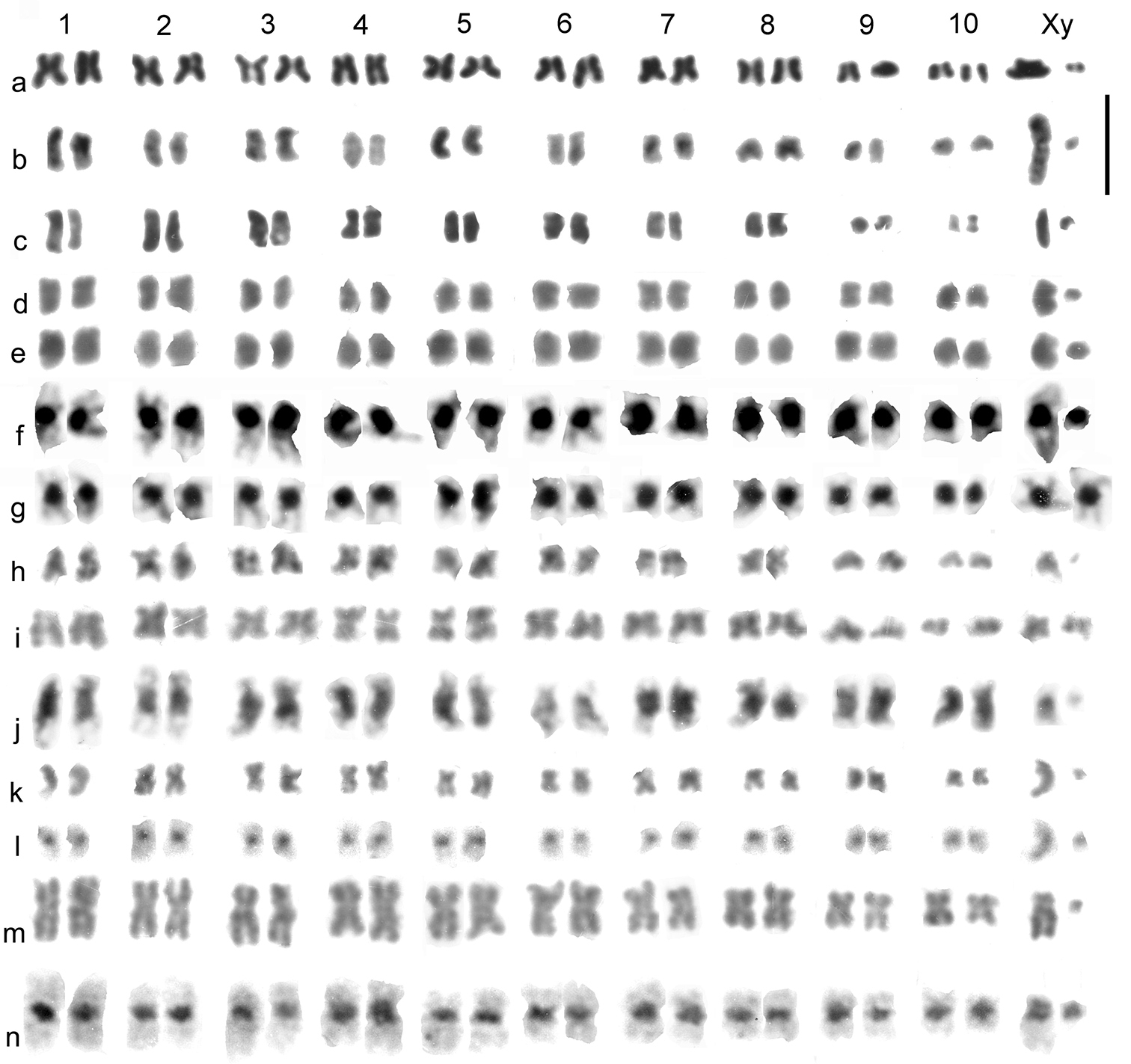
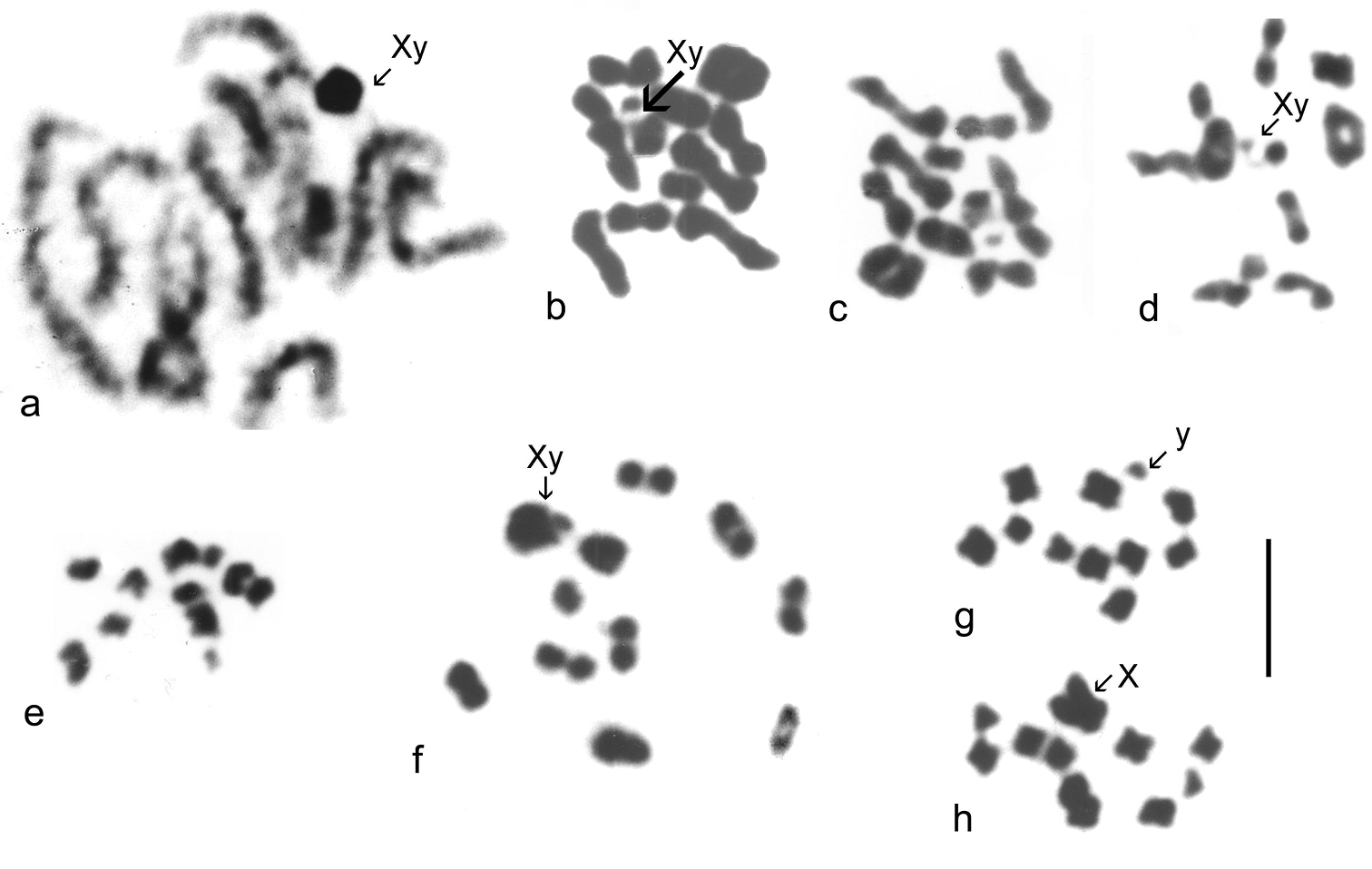
Catops coracinus. 2n = 20 + Xy (♂). Giemsa-stained mitotic chromosomes (unbanded), arranged as karyotypes are shown in Fig. 2 a (from mid-gut) and b (from testis). The autosomes are all submetacentric and show an even decrease in length so that pair 10 is about half the length of pair 1. The X chromosome is also submetacentric and is the longest chromosome in the nucleus, about twice the length of autosome 1. The y chromosome is small, almost dot-like. First metaphase of meiosis is shown in Fig. 5f, which shows the 10 autosomal bivalents and the large X chromosome associated with the y in the typical “parachute” association (Xyp) of Polyphaga (
Leiodes calcarata. 2n = 20 + Xy (♂). Unbanded Giemsa-stained mitotic chromosomes from testis are shown in Fig. 2c. The autosomes and X chromosome are all metacentric or submetacentric. The X chromosome is about the same size as autosome 1, which appears similar in size to that of Catops coracinus (compare Fig 1b and c). The autosomes show an even decrease in length from pairs 1 – 8, which pair 8 about half the length of pair 1. There is then an abrupt decrease to pairs 9 and 10, which are about half the length of pair 8. The y chromosome is dot-like. Zygotene of first division of meiosis is shown in Fig. 5a, where the heavily condensed Xy bivalent is distinct. Fig. 5b–d shows first metaphase, with the Xyp bivalent very clear. Fig 5 e shows a second metaphase nucleus, with the y chromosome clearly present.
Maps showing the collection sites of the material used in this paper (Nos 1–8), previously published material (Nos 9–18, see Table 2) and areas with carbonate rock outcrops in Cantabria, N Spain (squares 10 × 10 km). See Table 1 for explanation of numbers 1–8, and note that neighbouring sites may share the same number.
Mitotic chromosomes of Leiodidae, arranged as karyotypes: a, b Catops coracinus, unbanded a mid-gut b testis c Leiodes calcarata, testis d – g Cantabrogeus luquei, mid-gut d, e ♂ unbanded f ♂ C-banded g ♀ C-banded h, i Espanoliella luquei, mid-gut, unbanded h ♂ i ♀ j Fresnedaella lucius, testis, C-banded k, l Notidocharis uhagoni, ♂, mid-gut k unbanded l the same nucleus C-banded m, n Quaestus pasensis, mid gut m unbanded n the same nucleus C-banded. Scale bar = 5 µm.
Cantabrogeus luquei. 2n = 20 + Xy (♂), 20 + XX (♀). Unbanded Giemsa-stained mitotic chromosomes from mid-gut cells are shown as karyotypes in Fig 2 d, e (♂), and chromosomes from a C-banded ♂ mid-gut nucleus is shown in Fig. 2f. Fig. 2g shows chromosomes from a C-banded ♀ mid-gut nucleus. The chromosomes shown in Fig. 2 e and f are shown as found in Fig. 3a, b. All the autosomes, and the X chromosome are metacentric with heavy centromeric C-bands. The autosomes show an even decrease in size along the karyotype, with pair 10 about half the length of pair 1. The X chromosome, about 1.5 × the length of autosome 1, is the largest in the nucleus. The y chromosome is a relatively large dot. These preparations are completely consistent with one another and leave no doubt that 20 + Xy or XX is the true diploid number for this species.
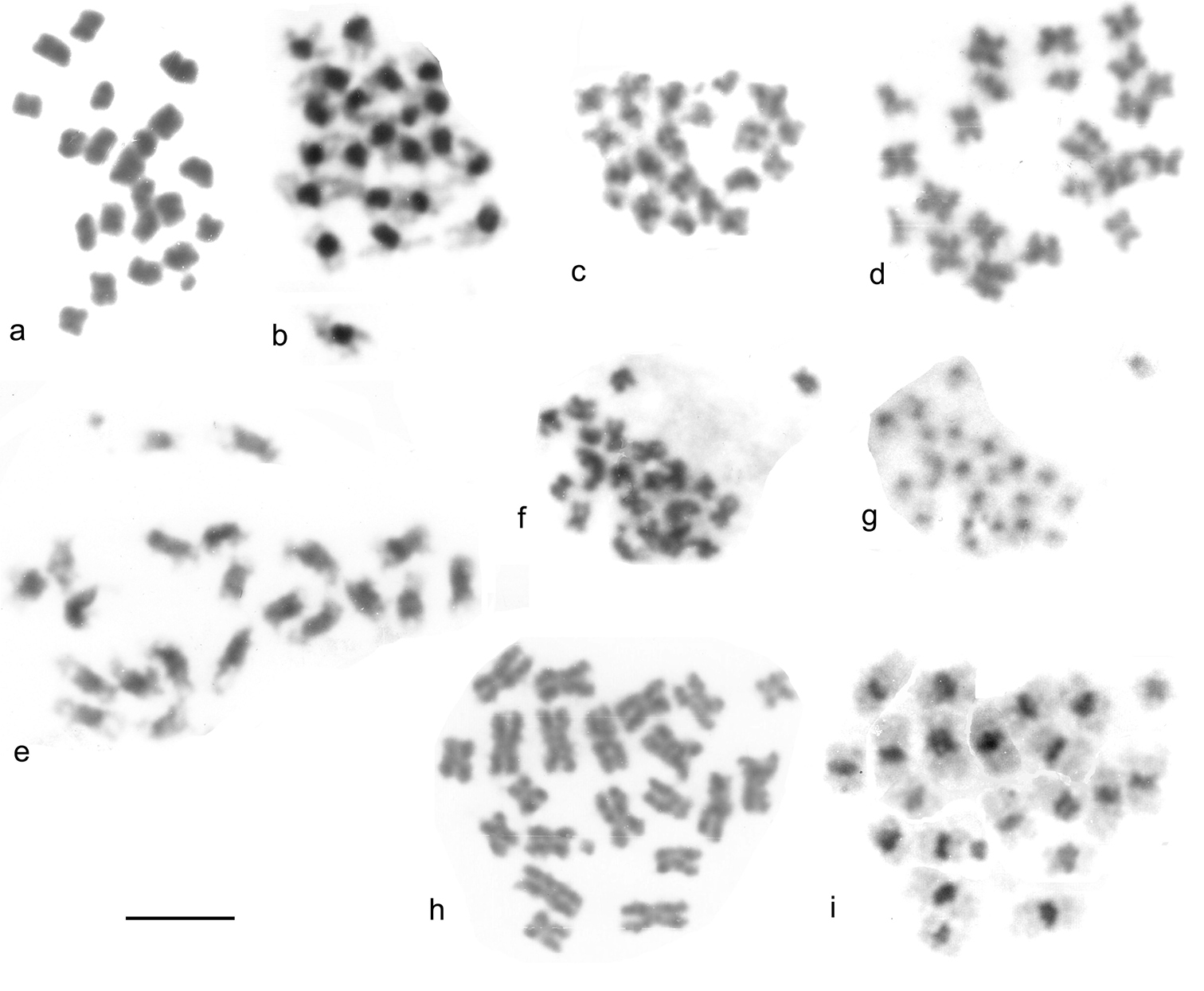
Giemsa stained mitotic chromosomes of Leptodorini as found. a, b Cantabrogeus luquei, mid-gut cell a plain b C-banded c, d Espanoliella luquei, mid-gut cells, plain c ♂ d ♀ e Fresnedaella lucius, testis, C-banded f, g Notidocharis uhagoni, ♂, mid-gut cell f plain g the same nucleus C-banded h, i Quaestus pasensis, ♂, mid-gut cell h plain i the same nucleus C-banded. Scale bar = 5 µm.
Espanoliella luquei. 2n = 20 + Xy (♂), 20 + XX (♀). Unbanded Giemsa-stained mitotic chromosomes from mid-gut cells are shown as karyotypes in Fig. 2h (♂) and i (♀), and as found in Fig. 3c, d. No C-banded preparation is available. The chromosomes in these preparations are all rather condensed, but appear either metacentric or submetacentric, with autosome pair 1 distinctly longer than the others, and a gradual decrease in length from pairs 2 – 10. The X chromosome appears similar in length to the medium-sized autosomes and the y chromosome is dot-like. As with Cantabrogeus luquei, the preparations are consistent with one another and leave no doubt that the chromosome number reported here is correct.
Fresnedaella lucius. 2n = 20 + Xy (♂), 20 + XX (♀). Fig. 2j shows spontaneously C-banded Giemsa-stained mitotic chromosomes from testis. Fig 3e shows these chromosomes as found. The female preparations (not shown) were from mid-gut, completely unbanded and with the chromatids beginning to separate following maximum contraction at metaphase. They are, however, adequate to confirm the chromosome number. Spontaneous C-banding was frequent among testis preparations from these Leptodirini, but in most cases the preparations were not adequate for preparation of karyotypes. The X chromosome is the smallest in the nucleus, apart from the dot-like y. The C-bands are particularly weak in autosome 6 and slightly weaker than most in autosome 2 and the X chromosome, but in the remaining autosomes they are very strong. All the autosomes and the X chromosome are metacentric to submetacentric.
Notidocharis uhagoni. 2n = 20 + Xy (♂). Fig. 2k, l shows karyotypes from a ♂ mid-gut cell, unbanded and C-banded. These chromosomes are shown as found in Fig. 3f, g. The autosomes and X chromosome are all metacentric or submetacentric, with small centromeric C-bands. The X chromosome, about twice as long as autosome 1, is the longest in the nucleus, and the autosome lengths decrease fairly evenly along the karyotype, with autosome par 10 about half the length of pair 1. The y chromosome is dot-like. As with the other species, the preparations are consistent and there is no reason to doubt the number obtained.
Quaestus pasensis. 2n = 20 + Xy (♂). Fig. 2m, n shows karyotypes from a mid-gut cell, unbanded and C-banded, and Fig. 3h, i shows these chromosomes as found, before and after C-banding. Autosome pair 3 and the X chromosome are more or less subacrocentric while the other autosome pairs are metacentric to submetacentric. The autosome pairs decrease in length evenly along the karyotype, with pair 10 slightly less than half the length of pair 1. The X chromosome is about as long as autosome pair 5 and the y chromosome is dot-like. The centromeric C-bands are distinct, with those of autosome pairs 1 and 4 about twice the size of the others. As with the other species, the preparations obtained are completely consistent with one another and leave no reason to doubt their accuracy.
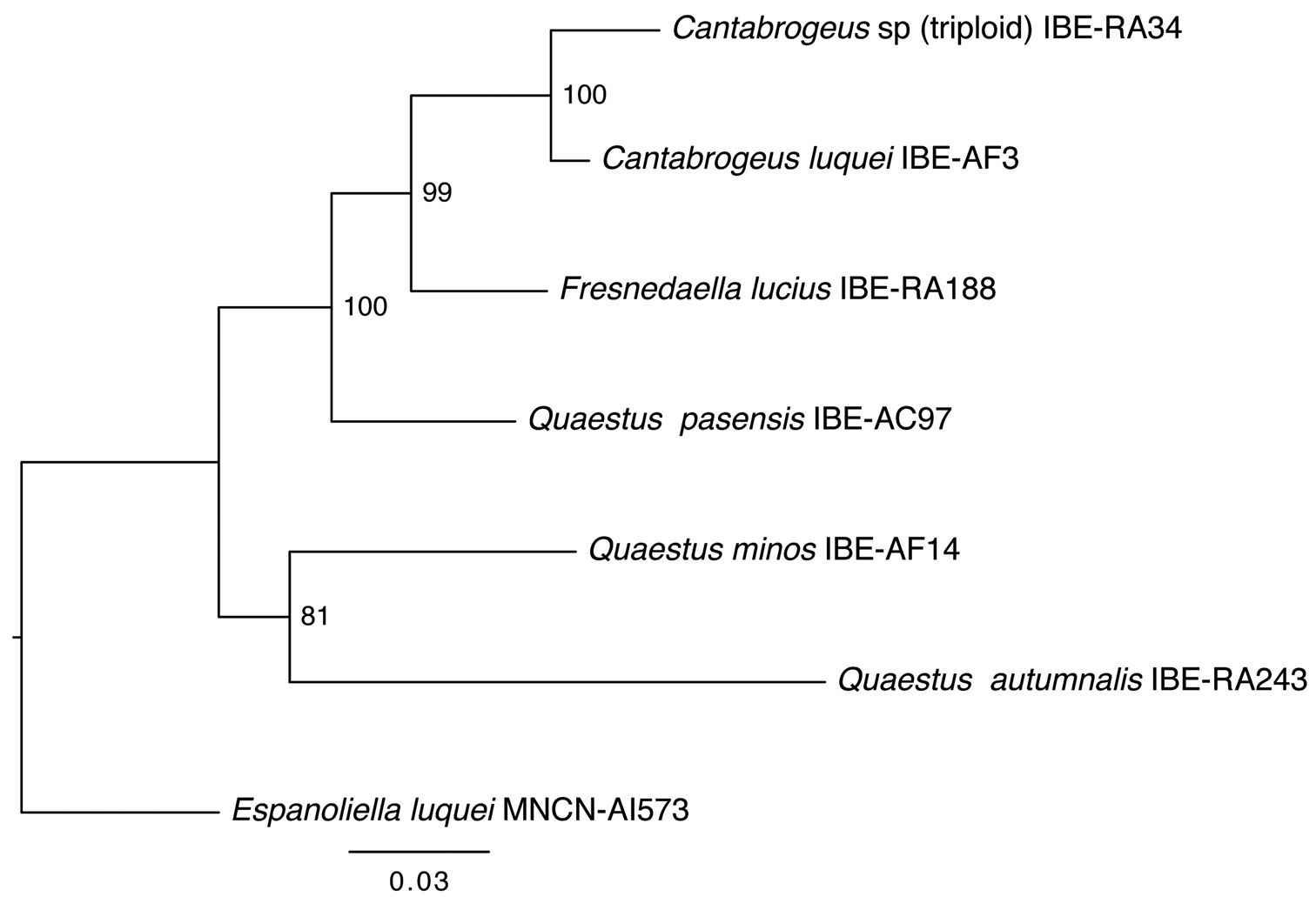
Triploid speciesCantabrogeus sp. (
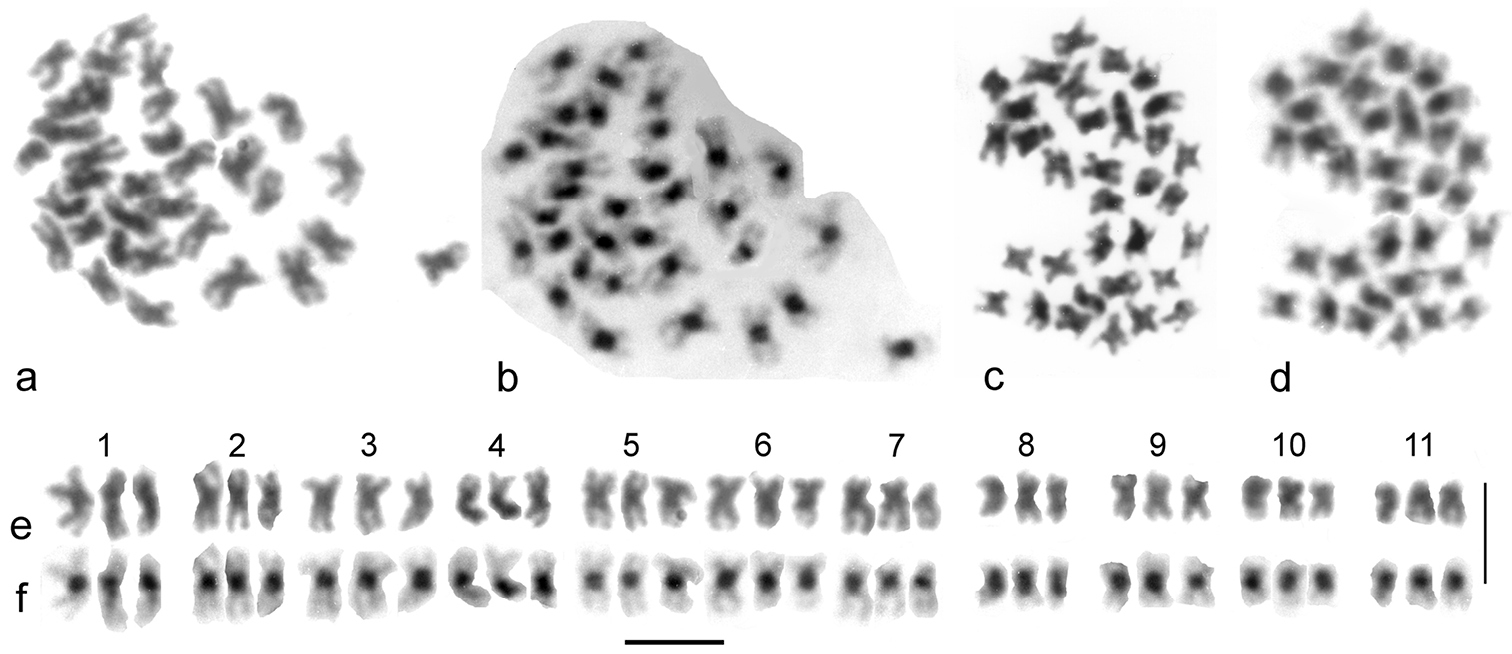
Cantabrogeus triploid species, mitotic chromosomes from mid-gut nuclei. a–d the chromosomes as found a, c unbanded b, d the same nuclei C-banded e, f karyotypes assembled from the nucleus figured in a & b. Scale bar = 5 µm.
Meiosis of Leiodidae (surface forms). a–e Leiodes calcarata f–h Catops coracinus a prophase I, zygotene b–d metaphase I e, metaphase II, ♂-determining, with y chromosome f metaphase I g, h metaphase II g ♂-determining, with y chromosome h ♀-determining, with X chromosome. Scale bar = 5 µm.
DNA from a specimen captured on June 6, 2009 (ref. IBE-RA34) was analyzed by I. Ribera (
Phylogenetic relationships of triploid Cantabrogeus sp., obtained with the same genes and methodology used by
As mentioned in the Introduction, the results obtained here appear both surprising and puzzling as all published data indicate a diploid chromosome number of 22 + Xy (♂), which
Species of Leptodorini for which chromosome details have been published: species with map No. As shown in Fig. 1, area from which the material was collected, and publication reference.
| Species (Map No.) | Area | Reference |
|---|---|---|
| Speonomus hydrophilus (Jeannel, 1908) (No. 9) | Pyrenees, France-Spain |
|
| Speonomus pyrenaeus (Lespès, 1857) (No.10) | ||
| Parvospeonomus delarouzeei (Fairmaire, 1860) (No.11) |
|
|
| Troglocharinuselongatus Zariquiey, 1950(=variabilis Bellés, 1978)(No. 12) | ||
| Troglocharinuselongatus jacasi (Lagar, 1966) (No. 13) | ||
| Troglocharinuselongatus schibii (Español, 1972) (No. 14) | ||
| Troglocharinuselongatus ferreri (Reitter, 1908) subspecies pallaresi Bellés, 1973 (No. 15) | ||
| Troglocharinuselongatus kiesenwetteri (Dieck, 1869) (No. 16) | ||
| Pholeuon knirschi Breit, 1911 (No. 17) | Carpathians, Romania |
|
| Drimeotus kovacsi Miller, 1856 subspecies viehmanni Ieniştea, 1955 (No. 18) |
Phylogram obtained from
The cave-dwelling specimens referred to in this paper were collected during several biospeleological investigations authorized and funded by the Government of Cantabria (Spain). The studies and explorations in Cantabria were supported in part by grants from the General Direction for Biodiversity of the Regional Government of Cantabria. We thank J.M. Salgado (University of León, Spain), for assistance in collecting specimens, and I. Ribera (Institute of Evolutionary Biology, CSIC–UPF, Barcelona, Spain) who kindly provided information about molecular studies.
The chromosomal work was carried out in the Natural History Museum, London and the School of Biological Sciences, Royal Holloway, University of London, whom we thank for the facilities to carry out these investigations.