(C) 2012 Sergey N. Matveevsky. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Immunocytochemical and electron microscopic analysis of synaptonemal complexes (SCs) was carried out for the first time in homozygotes and complex Robertsonian heterozygotes (hybrids) of the common shrew, Sorex araneus Linnaeus, 1758, from a newly discovered hybrid zone between the Moscow and the Neroosa chromosomal races. These races differ in four monobrachial homologous metacentrics, and closed SC tetravalent is expected to be formed in meiosis of a hybrid. Indeed, such a multivalent was found at meiotic prophase I in hybrids. Interactions between multivalent and both autosomes and/or the sex chromosomes were observed. For the first time we have used immunocytochemical techniques to analyse asynapsis in Sorex araneus and show that the multivalent pairs in an orderly fashion with complete synapsis. Despite some signs of spermatocytes arrested in the meiotic prophase I, hybrids had large number of active sperm. Thus, Moscow – Neroosa hybrid males that form a ring-of-four meiotic configuration are most likely not sterile. Our results support previous demonstrations that monobrachial homology of metacentrics of the common shrew does not lead to complete reproductive isolation between parapatric chromosomal races of the species.
Synaptonemal complex, MSCI, γH2AX, centromeres, Sorex araneus
The concept of chromosomal speciation implies occurrence of reproductive isolation as a result of chromosomal rearrangements (
Due to its high level of karyotype variability, the common shrew Sorex araneus is subdivided into at least 72 parapatric chromosomal races, each characterised by a unique set of metacentric chromosomes formed by Robertsonian fusions and/or whole-arm reciprocal translocations (WARTs) (
Hybrids between parapatric chromosomal races of the common shrew are often expected to be complex Robertsonian heterozygotes with monobrachial homology, which form chain (C) or ring (R) configurations of three or more elements at prophase I of meiosis. Such complex meiotic configurationsare considered to be more susceptible to irregularity. As a consequence, complex heterozygotes are expected to be less fertile than homozygotes of pure chromosomal races. At present, interracial hybrids with different types of meiotic configurations from CIII and RIV up to CXI and RVI have been revealed from seventeen well-studied hybrid zones (
A new chromosomal hybrid zone between the Moscow race (gm, hi, jl, kr, no, pq, 2na=18) and the Neroosa race (go, hi, jl, kr, mn, pq, 2na=18) has been found recently in the centre of European Russia (
This paper presents a comparative synaptonemal complex (SC)analysis of prophase I of meiosis using electron microscopy and immunofluorescence in homozygotes and complex Robertsonian heterozygotes from this hybrid zone. A combination of both methods together for SC analysis is used for the first time in Sorex araneus.
Material and methodsAnimals and karyotypes. A total of eight adult male common shrews were collected from the Moscow-Neroosa hybrid zone, located in the south-eastern part of the Moscow Region near Ozyory town (the left bank of the River Oka), in April 2012, at the beginning of the breeding season. Each specimen was processed according to the field procedure described in
Synaptonemal Complex Analysis. Synaptonemal complex (SC) preparations were prepared and fixed using the technique of
Electron microscopy. Slides were stained with a 50% AgNO3 solution in a humid chamber for 3 h at 56°C, washed 4 times in distilled water and air dried. Stained slides were observed under a light microscope to select suitably spread cells. Once selected, plastic (Falcon film) circles were cut out with a diamond tap and transferred onto grids and examined in a JEM 100B electron microscope.
Immunofluorescence. Poly-L-lysine-coated glass was used for immunostaining. The slides were placed in phosphate-buffered saline (PBS) and incubated overnight at 4°C with the following primary antibodies diluted in antibody dilution buffer (ADB: 3% bovine serum albumin - BSA, 0.05% Triton X-100 in PBS): rabbit anti-SCP3 1:200 (Abcam, Ab15093), human anti-centromere antibodies, ACA 1:200 (Antibody Incorporated, 15-235) and mouse anti-phospho-histone γH2AX 1:500 (Abcam, Ab26350). After rinsing in PBS (3 times for 10 min), the slides were incubated with appropriate secondary antibodies diluted 1:800 in PBS: goat anti-rabbit Alexa Fluore 488 conjugated antibodies, goat anti-human Alexa Fluore 546 conjugated antibodies and FITC-conjugated horse anti-mouse IgG (all Abcam) at 37°C for 90 min. After a final rinse in PBS, the slides were mounted in Vectashield with DAPI (Vector Laboratories). Slides were analyzed in an Axioimager D1 microscope CHROMA filter sets (Carl Zeiss, Jena, Germany) equipped with an Axiocam HRm CCD camera (Carl Zeiss), and image-processing AxioVision Release 4.6.3. software (Carl Zeiss, Germany). Images were processed using Adobe Photoshop CS3 Extended.
ResultsKaryotypes. Three of the eight karyotyped shrews were complex heterozygotes, i.e. F1 hybrids. They showed the expected arm combinations of Rb metacentrics - go/gm/mn/no, hi, jl, kr, pq. Five other shrews were homozygotes with Moscow race karyotype (gm, hi, jl, kr, no, pq). Hybrid individuals and homozygotes of the pure race had 2n=21, NF=40, XY1Y2. Only two hybrids and one homozygote were subject to comparative SC analysis.
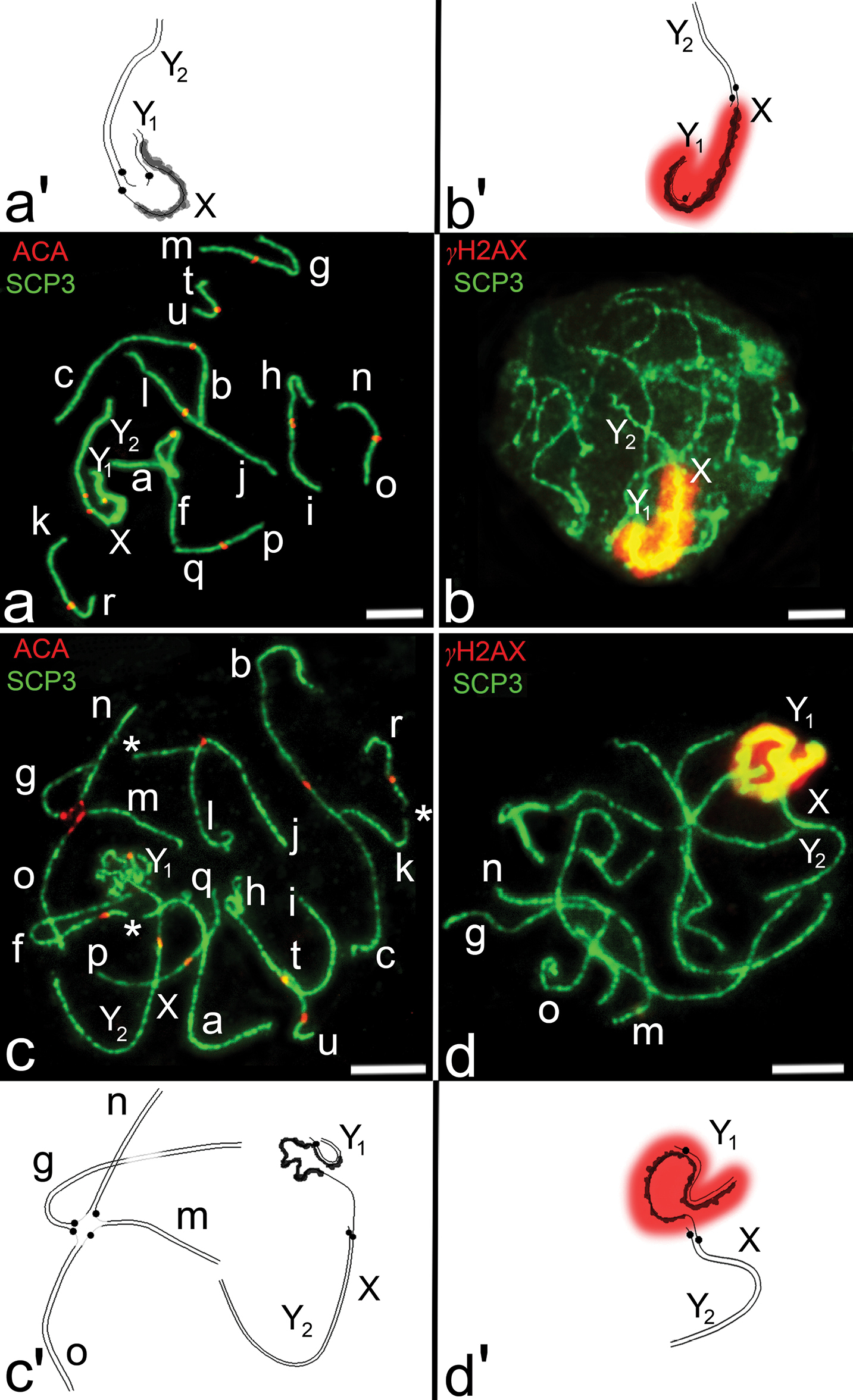
SC analysis of a homozygote of the common shrew. Immunocytochemical analysis of SCs in pachytene spermatocytes of the homozygote revealed nine SC bivalents (af, bc, jl, hi, gm, no, kr, pq, tu) and the sex trivalent (XY1Y2), as expected from the G-banded karyotype of the Moscow race. Centromeres of hi SC bivalent and centromeres in the sex trivalent were not aligned. The sex trivalent exhibited irregular thickenings of the “true” arm of the X chromosome. The autosomal arm of the X chromosome formed a typical SC (Fig. 1a, a’). γH2AX covered only the synaptic region of the X and the Y1 chromosomes and the thickened part of the X chromosome. The autosomal arm of the X chromosome is not involved in inactivation (Fig. 1b, b’).
SC analysis of complex heterozygotes of the common shrew. As expected, seven SC bivalents (af, bc, jl, hi, kr, pq, tu), an SC tetravalent (g/o/n/m) and the sex trivalent XY1Y2 were detected in spermatocyte nuclei at pachytene stage (Fig. 1c, c’, 2). According to the previously elaborated classification, the SC tetravalent represents a closed SC multivalent which was formed due to monobrachial homology (
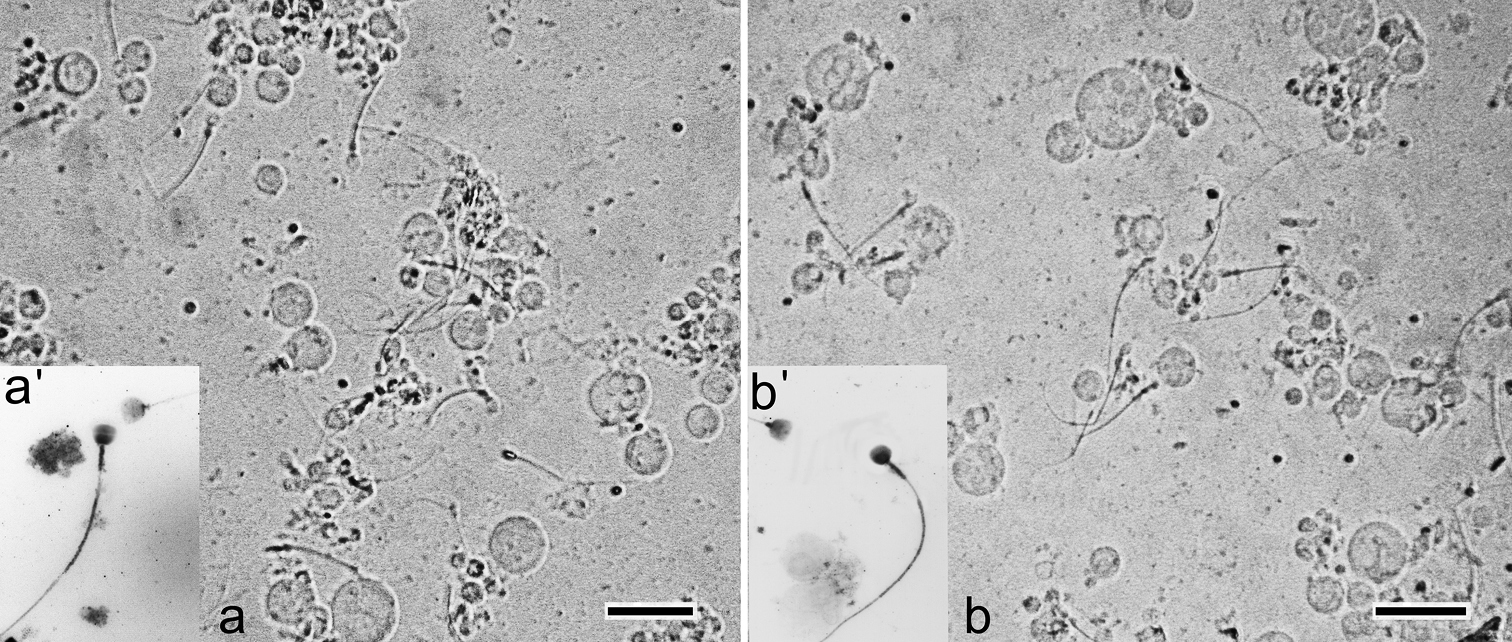
Suspension of testis cells. There are spermatocytes and active spermatozoa in testis cell suspension from common shrews of both Moscow race and hybrids (Fig. 3). Chromosome spreads also contained a significant amount of spermatozoa (Fig. 3a’, b’).
a–d Synaptonemal complexes of homozygotes and complex heterozygotes of the common shrew. Immunostaining with antibodies against axial elements of SC - SCP3 (green), polyclonal antibodies to centromeric protein ACA (red) and antibodies to γH2AX (red) marking chromosome asynaptic regions. Bar = 5 μm a, b SCs from spermatocyte pachytene nuclei (the Moscow race) a Nine SC bivalents (af, bc, jl, hi, gm, no, kr, pq, tu) and sex trivalent XY1Y2. Sex trivalent contains irregular thickening of the “true” arm of X-chromosome (scheme a’). The autosomal arm of the X-chromosome forms a typical SC. Centromeres within hi bivalent and XY1Y2 trivalent are displaced relative to each other b Anti-γH2AX antibodies recognize chromatin in the synaptic zone of X and Y1 chromosomes and unsynapsed thickened region of the “true” arm of X-chromosome (scheme b’) c, d SCs from spermatocyte pachytene nuclei obtained from Moscow-Neroosa hybrids c Seven SC bivalents (af, bc, jl, hi, kr, pq, tu), sex trivalent XY1Y2 and SC tetravalent (g/o/n/m) were revealed in spermatocyte nuclei of complex heterozygotes. Gaps were detected in SC bivalents af, kr and in g arm of SC-tetravalent (indicated with asterisks). Gaps were also detected in pericentromeric regions of all metacentrics of the SC tetravalent (scheme c’). af SC bivalent is associated with sex trivalent; d Anti-γH2AX antibodies identify chromatin in the synaptic region of X and Y1 chromosomes and asynaptic thickening of the “true” arm of the X-chromosome (scheme d’), as for common shrew spermatocytes from Moscow race (Fig. 2b). One of the SC bivalents is associated with the true part of sex trivalent. The SC tetravalent is usually associated with one or two autosomes (c, d).
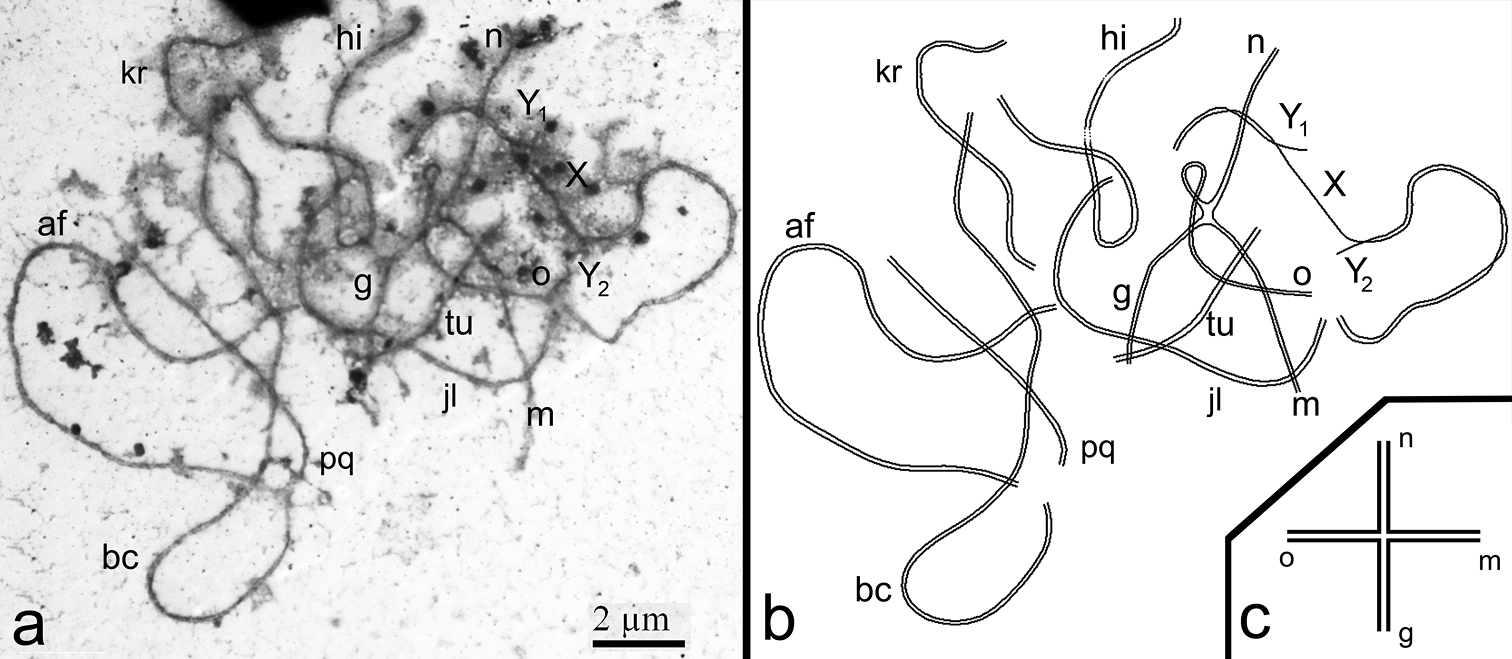
a–d A pachytene spermatocyte of the Moscow-Neroosa hybrid. a An electron micrograph. Seven SC bivalents (af, bc, jl, hi, kr, pq, tu), the sex trivalent XY1Y2 and the SC tetravalent (g/o/n/m) are detected. Closed SC tetravalent is composed of four monobrachial homologous metacentrics go, on, nm, mg. SC tetravalent is associated with two autosomes and sex trivalent. Bar = 2 μm b A scheme of chromosome synapsis on the basis of Fig. 2a c A scheme of SC tetravalent.
a–b Cell suspension of common shrew testis a – homozygote (the Moscow race) b – complex heterozygote from interracial hybrid zone a’, b’ Inverted image of spermatozoa (non-specific binding of anti-SCP3 antibodies after immunocytochemistry). Bar = 20 μm.
Hybrid zones of Sorex araneus represent unique natural laboratories for studying Robertsonian chromosomal polymorphism. Complex cytogenetic studies have been carried out in 17 known chromosomal hybrid zones; however only seven of them have been subjected to analysis of early stages of meiosis including synaptonemal complexes analysis (see Table 1). Such studies provide information about the peculiarities of chromosomal synapsis and separation of multivalents in meiosis, which determine a hybrid’s sterility/fertility. The latter is important for estimation of reproductive isolation level between different chromosomal races.
The model of chromosomal speciation by monobrachial centric fusion has been proposed by
It was assumed that interracial hybrids of the common shrew (complex heterozygotes with multiple Rb rearrangements) had either significantly reduced fertility or were completely sterile (
Previous studies have also demonstrated that association of autosomes and complex SC configurations with sex chromosomes in meiotic prophase I could cause reduction of fertility or even complete sterility (
To reveal the signs of defects in spermatogenesis in our specimens, we studied the dynamics of meiotic prophase I focusing on the sex trivalent. Normally, sex chromosomes of male mammals contain a short SC in pseudoautosomal region and long unpaired axes in meiotic prophase I. Also, sex chromosomes often move to the periphery of pachytene nuclei and undergo MSCI (meiotic sex chromosome inactivation), which is required for successful progression of meiosis (
We found that the behavior of the sex trivalent in homozygotes (Moscow race) was similar to that of sex chromosomes in meiotic prophase I in other mammals. However, in complex heterozygotes, the sex trivalent interacted with autosomes in some prophase nuclei, which was typical of hybrids and heterozygotes with chromosomal rearrangements and reduced fertility (
Formation of complex SC configurations is known to be associated with a high degree of asynapsis. In such cases, chromosome asynaptic regions undergo transcriptional inactivation MSUC (meiotic silencing of unsynapsed chromatin), which in its turn results in meiotic arrest and reduction of fertility (
Probably, the four metacentrics that form SC tetravalent in interracial Moscow-Neroosa hybrids undergo successful separation, spermatocytes are not arrested (or are arrested partially) and balanced gametes are formed in the end. This is also supported by the presence of numerous spermatozoa in hybrid testis cell suspensions. Further studies are needed to measure the level of aneuploidy.
Our data conform to the results of other authors. For example, no defects of sex body formation were detected in most spermatocyte nuclei of mice that were heterozygous for eight Rb translocations, which indicated moderate activity of pachytene arrest (
In our study, centromeres of homologues in the SC bivalent formed between two Rb metacentrics (hi) were not aligned. We assume that this pattern might result from different mechanisms of Rb metacentric formation in the past. One of the ancestors might have retained centromere of h chromosome after formation of Rb metacentric, while another ancestor might have retained centromere of i chromosome. Previous works reported a presence of two centromeric foci in other Rb metacentrics (
Our synaptonemal complex results suggest regularity in formation of the ring-of-four configuration produced by hybrids between the Moscow and the Neroosa chromosome races of Sorex araneus. This relates well to the previous findings that chromosome races differing by monobrachial homology in Sorex araneus does not lead to complete sterility in hybrids. In particular, our immunocytochemical demonstration of an absence of asynapsis in the ring-of-four configuration relates well to the production of sperm in such hybrids.
SC analysis in chromosomal hybrid zones of the common shrew.
| Hybrid zone | Examined karyotypic categories | Detected SC-configuration | Reference |
|---|---|---|---|
| Oxford Hermitage | SH (chain-of-three) (k/q), (n/o), (p/r) | SC trivalents |
|
| Oxford Wirral | SH (chain-of-three) (k/q), (n/o), (k/o), (j/l) | SC trivalents |
|
| Abisko Sidensjö | CH (chain-of-four) i/ih/hn/n | SC tetravalent |
|
| Aberdeen/ Oxford | CH (chain-of-seven) r/rp/pn/no/ok/kq/q | SC chain with 7 elements |
|
| Novosibirsk/Tomsk | CH (chain-of-eight) + (chain-of-three) o/og/gk/ki/ih/hn/nm/m, q/r | SC chain with 8 elements and SC trivalent |
|
| Moscow Seliger | CH (chain-of-eleven) g/gm/mh/hi/ik/kr/rp/pq/qn/no/o | SC chain with 11 elements |
|
| Uppsala Hällefors | CH (ring-of-four) qp/pk/ko/oq | SC tetravalent |
|
| Moscow Neroosa | CH (ring-of-four) og/gm/mn/no | SC tetravalent | this study |
SH – simple heterozygotes, CH – complex heterozygotes
We would like to thank Dr Nina Bulatova for discussion on the structure of the manuscript and valuable comments in karyology. We are especially grateful to Prof Jeremy B. Searle for valuable comments on the manuscript and the English. This work was supported by President Grant for Russian Distinguished Young Scientists MK-2500.2011.4 (for SP) and research grants of the Russian Foundation for Basic Research № 12-04-31425, 12-04-31200.