(C) 2012 Oleg S. Alexandrov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Humulus japonicus Siebold et Zucc (Japanese hop) is a dioecious species of the family Cannabaceae. The chromosome number is 2n = 16 = 14 + XX for females and 2n = 17 = 14 + XY1Y2 for male. To date, no fluorescence in situ hybridization (FISH) markers have been established for the identification of Humulus japonicus sex chromosomes. In this paper, we report a method for the mitotic and meiotic sex chromosome differentiation in Humulus japonicus by FISH for HJSR, a high copy subtelomeric repeat. The signal is present in the subtelomeric region of one arm of the X chromosome. We demonstrate that males have two Y chromosomes that differ in FISH signal with the HJSR probe. Indeed, the HJSR probe hybridizes to a subtelomeric region on both arms of chromosome Y1 but not of chromosome Y2. The orientation and position of pseudoautosomal regions (PAR1 and PAR2) were also determined.
Humulus japonicus, sex chromosomes, sex determination in plants, subtelomeric repeat, fluorescence in situ hybridization
Humulus japonicus Siebold & Zuccarini, 1846 (Japanese hop) is a dioecious, climbing and annual species of the family Cannabaceae. The chromosome number is 2n = 16 = 14 + XX for females and 2n = 17 = 14 + XY1Y2 for males. The sex of Humulus japonicus is determined by the ratio of X chromosomes and autosomes sets (A); a X:A ratio of 1.0 results in a female and a ratio of 0.5 results in a male (the Y chromosomes are dispensable) (
The closest relative of Humulus japonicus is the common hop Humulus lupulus Linnaeus, 1753. H. lupulus has the same sex determination system as in Japanese hop (X/A) but it differs in chromosome number (2n = 20 in both female and male plants) and in sex chromosome systems (XX/XY) (
Due to the great economic importance of Humulus lupulus, molecular methods to assess genetic variability and genome organisation have been developed for this species. To understand sex chromosome evolution and organisation in plants, sex-linked genetic and cytogenetic markers are required. Male-specific DNA markers have been identified in Humulus lupulus (
Cytogenetic markers of Humulus lupulus sex chromosomes were established by application of C-banding/DAPI (
A trivalent formation comprising Y1-X-Y2 associated with terminal chiasmata has been observed during meiosis in Humulus japonicus (
To date, no FISH markers have been established for the identification of Humulus japonicus sex chromosomes. In this paper, we report a method for sex chromosome differentiation in Humulus japonicus by FISH with the subtelomeric repeat HJSR on mitotic and meiotic chromosomes.
Material and methodsMale and female plants of Humulus japonicus raised from seeds of cv. Samuray (“Gavrish seeds”, Moscow, Russia) and seed lot № 4 (“Flos”, Moscow, Russia) were used in this study.
Total genomic DNA was isolated from young leaf material using the CTAB method (
Mitotic metaphase chromosomes were prepared from fast growing root tip meristems collected from plants. They were pre-treated in 0.01 % α-bromonaphtalene at 4°C for 24 h and fixed in 3:1 (v/v) 96% ethanol: glacial acetic acid at room temperature for 1 h. For preparation of the microscopic slides, the root tips were rinsed in running water for 1 h and in distilled water three times and then were incubated in a 10 mM citrate buffer (pH 4.9) containing 0.4 % cellulase Onozuka R10 (Serva, Germany) and 0.2 % pectolyase Y-23 at 37°C for 3 h. Afterwards, the macerated root tips were spread by dissecting the tissue in 60 % acetic acid and by squashing it under a coverslip.
For meiotic chromosome preparations, the young anthers about 3-5 mm long at metaphase I were collected and fixed directly in acetic-ethanol (1:3) for 1 h, rinsed in water and then incubated for 2–3 hours in pectolytic enzymes containing 0.8 % cellulase Onozuka R10 (Serva, Germany) and 0.4 % pectolyase Y-23 in a 10 mM citrate buffer (pH 4.9). After two washes in distilled water, the anthers were carefully transferred onto grease-free slides, and the pollen mother cells were dissected out of the anther into a 1 μl droplet of water. Then, 5 μl of 60 % acetic acid was added, and the pollen mother cells were left for 2–3 minutes until the cytoplasm became sufficiently clear. The cells were then squashed under a coverslip.
For fluorescence in situ hybridization (FISH), the plasmid with the Humulus japonicus HJSR subtelomeric repeat DNA was labelled with dioxigenin-11-dUTP. The 1 μg sample of the purified DNA was labelled by nick translation according to the manufacturer’s protocol (Roche Diagnostics Gmbh, Germany). The chromosome and probe denaturation as well as hybridization and posthybridization washes were performed as described by
For detection of Arabidopsis-type telomere repeat in Humulus japonicus chromosomes sequential FISH was applied. Cover glasses were carefully removed after by washing for 1 h with 0.2 % Tween 20. Probe DNA was dissociated from the chromosomes with 70 % formamide in 2×SSC for 5 min. Slides were the dehydrated for 3 min each of 70, 90 and 100 % (v/v) ethanol, and air-dried. A new hybridization mix was added to the slides. The Arabidopsis-type telomere probe used was the deoxyribinucleotide oligomer (5’-CCCTAAA-3’)3 synthesised with a TAMRA label (ZAO “Syntol”, Moscow, Russia) at the 5’ end. The chromosome preparations were counterstained with DAPI.
The slides were observed under an AxioImager.M1 fluorescent microscope, photographed with a monochrome AxioCam MRm CCD camera, and visualised using Axiovision software (Carl Zeiss). In each experiment, at least 35 chromosome plates were analysed.
ResultsThe isolated and cloned HJSR KpnI-repeat was sequenced and found to be 380 bp in length (GU831573). No sex specific differences have been found between the sequences of male and female plants. The consensus sequence of 380 bp fragment is 63.4 % AT and does not possess any direct or inverted sequences of significant length. The BLAST analysis did not reveal any significant homology with sequences of other organisms.
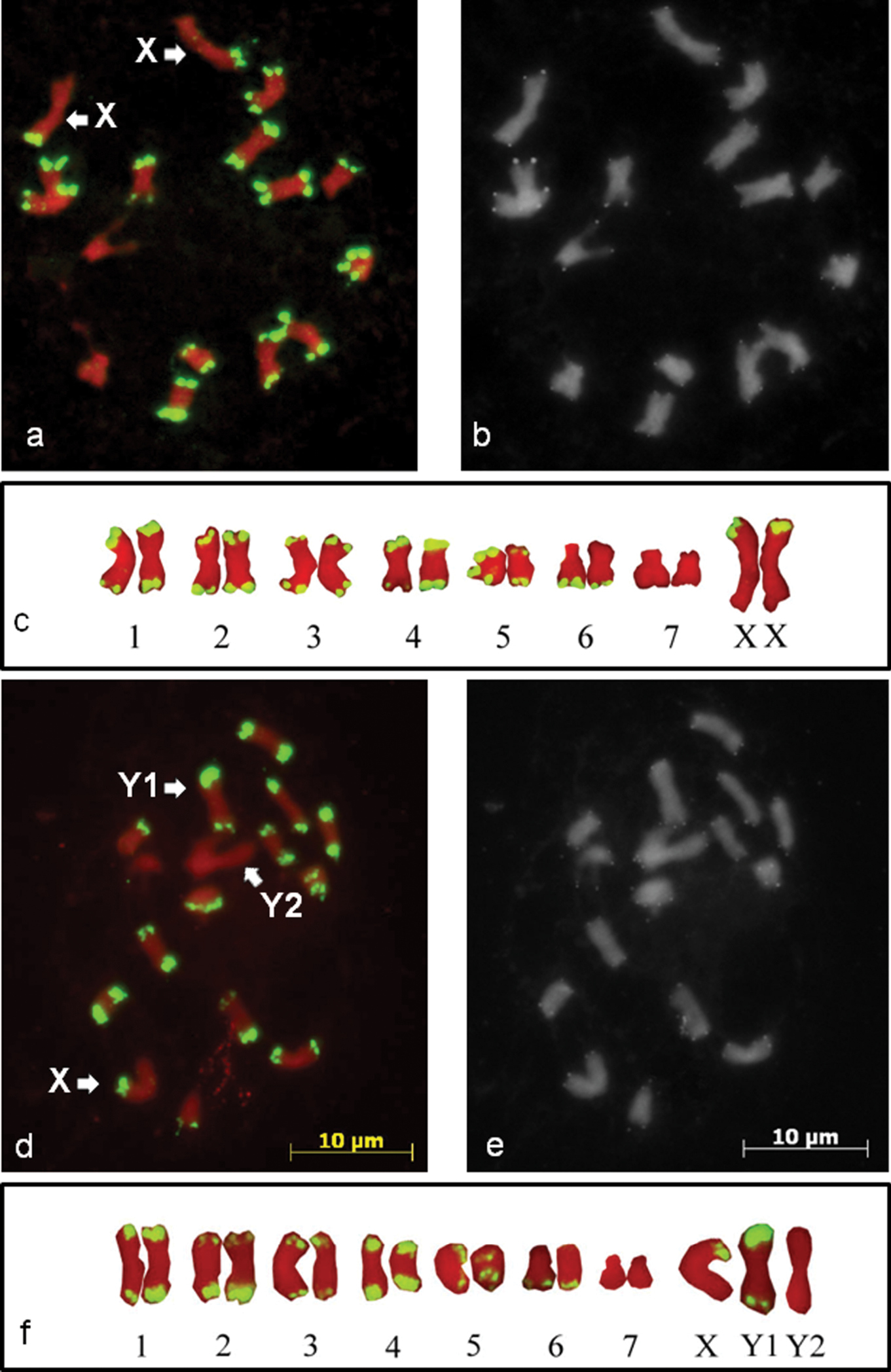
The FISH signals observed with the HJSR probe were localised to subtelomeric regions of the chromosomes, and the signals were observed at one or both distal ends of each chromosome in both males and females. However, the signal was completely absent on one pair of autosomes from males and females and additionally on one of the three biggest chromosomes from males (Fig. 1). The FISH signal colocalised with the subtelomeric DAPI positive bands. No signal was detected from the interstitial regions of the chromosomes. The metaphase plates of the male and female plants were compared and revealed that the female metaphase plates carry two X chromosomes with the HJSR repeat signal on one of the arms (Fig. 1a, c). The male metaphase plates appeared to possess chromosome X with one signal, chromosome Y1 with signals on both arms and chromosome Y2 with no signal (Fig. 1d, f). FISH of the mitotic chromosomes of Humulus japonicus with a probe for an Arabidopsis-type telomere repeat showed signals on the all chromosome ends of both male and female plants (Fig 1b, e). The locations of the FISH signals from the telomeric probe were more distal from the centromere than those with the HJSR probe. No interstitial Arabidopsis-type telomere repeat signals were observed on the chromosomes.
The mitotic chromosomes of Humulus japonicus. The chromosomes are counterstained by propidium iodide (red). The high copy HJSR subtelomeric repeat (green) is mapped to the female (a) and male (d) mitotic chromosomes of Humulus japonicus by FISH. The X, Y1 and Y2 chromosomes are marked by arrows. Sequential FISH with the Arabidopsis-type telomeric repeat on metaphase chromosomes of female (b) and male plants (e). The katyotypes of female (c) and male (f) plants.
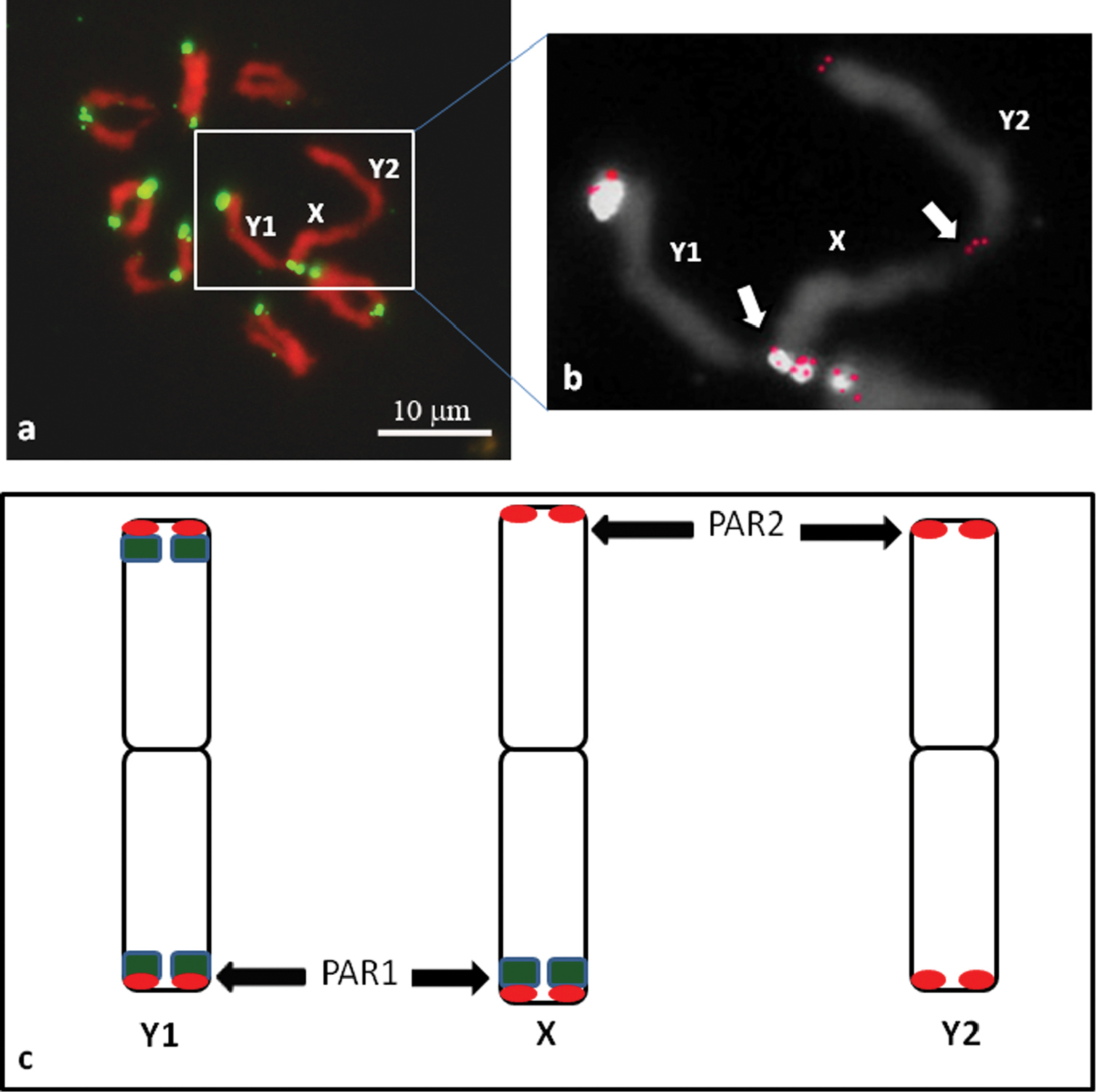
The results of the mitotic metaphase plate analyses are in agreement with the physical mapping of the HJSR to the meiotic chromosomes at diakinesis (Fig. 2). We identified the Y1-X-Y2 trivalent formation (Fig. 2a, b). The different ends of the X chromosome pair with different Y chromosomes. The Y1 chromosome, revealed HJSR FISH signals on both arms, pairs with arm of the X chromosome also carrying HJSR FISH signal. The Y2 chromosome has no HJSR FISH signal and pairs with the X chromosome arm that lacks a signal. This finding allows us to conclude that the pseudoautosomal regions (PAR1 and PAR2) are located at distal parts of both arms of the X chromosome and distally on one arm of each Y chromosome (Fig. 2c).
The meiotic chromosomes of Humulus japonicus at diakinesis with FISH signals for the HJSR repeat (green). The trivalent Y1-X-Y2 formation and chiasmata between the sex chromosomes can be clearly observed (a). The trivalent Y1-X-Y2 formation from (a) with combined signal of Arabidopsis-type telomeric repeat after sequential FISH (red) (b). Schematic diagram of the Humulus japonicus X, Y1 and Y2 chromosomes (c) with the hybridization of the HJSR probe (green) and the Arabidopsis-type telomeric repeat probe (red). The pseudoautosomal regions (PAR1 and PAR2) are indicated by the arrows.
Most satellite DNAs are specific at the species or species subgroup levels (
The orientation of the pseudoautosomal regions on the X chromosome indicates the important role of subtelomeric repeats in sex chromosome genesis. The nature of the Y chromosomes of Humulus is puzzling. The unusual sex chromosome system XX/XY1Y2 in Humulus japonicus points to the role of chromosome translocations in the karyotype evolution of this species. According to Ohno’s (1967) hypothesis, multiple sex chromosomes have evolved from the standard XX/XY systems by interchanges between autosomes and sex chromosomes. This has been shown in Silene diclinis (Lag.) M. Laínz, 1963 (
This work was supported by grant # P1164 NK-602P/7 from the Russian Ministry of Science and Education.