(C) 2012 I.A. Gavrilov-Zimin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of scale insects, Aclerda pseudozoysiae sp. n., is described and illustrated. The karyotypes and some aspects of reproductive biology and cytogenetics of the new species species and Aclerda takahashiiKuwana, 1932 were studied, representing the first data for the genus Aclerda Signoret, 1874 and the family Aclerdidae as a whole. Aclerda pseudozoysiae sp. n. has 2n=16, bisexual reproduction, and heterochromatinization of one haploid set of chromosomes in male stages of the life cycle, matching either a Lecanoid or a Comstockioid genetic system. Aclerda takahashii demonstrates 2n=18 and unusual type of parthenogenesis with diploid and haploid embryos (inside each gravid female) without heterochromatinization. Both species are ovoviviparous; all stages of embryonic development occur inside the mother’s body.
Scale insects, Aclerdidae, Aclerda takahashii, Aclerda pseudozoysiae, taxonomy, morphology, new species
The scale insect family Aclerdidae currently includes 5 genera with 58 species (ScaleNet <http://www.sel.barc.usda.gov/scalenet/scalenet.htm> –
The species of the family have never been specially studied. However,
Aclerda pseudozoysiae sp. n. K 884, Indonesia, New Guinea (Irian Jaya), vicinity of Jayapura city, slopes of Cyclop mountains above Entrop, dry primary forest interrupted by agricultural crops and sandy burrows, under the leaf sheaths of undetermined grass (Poaceae), 1.XI. 2011, Ilya Gavrilov-Zimin.
Aclerda takahashii. K 933, Indonesia, South-Eastern Sulawesi, vicinity of Kendari city near Haluoleo airport, chaotic agricultural plantations after recent deforestation, under the leaf sheaths of Saccharum sp., 12.XI.2011, Ilya Gavrilov-Zimin.
All material, including the types of the new species, is preserved in the Zoological Institute, Russian Academy of Sciences, St. Petersburg.
The chromosomal plates were prepared using a squash method in a drop of lactoacetorcein as previously described (
urn:lsid:zoobank.org:act:00A57BC1-DD95-4220-A601-C1554AFEBB87
http://species-id.net/wiki/Aclerda_pseudozoysiae
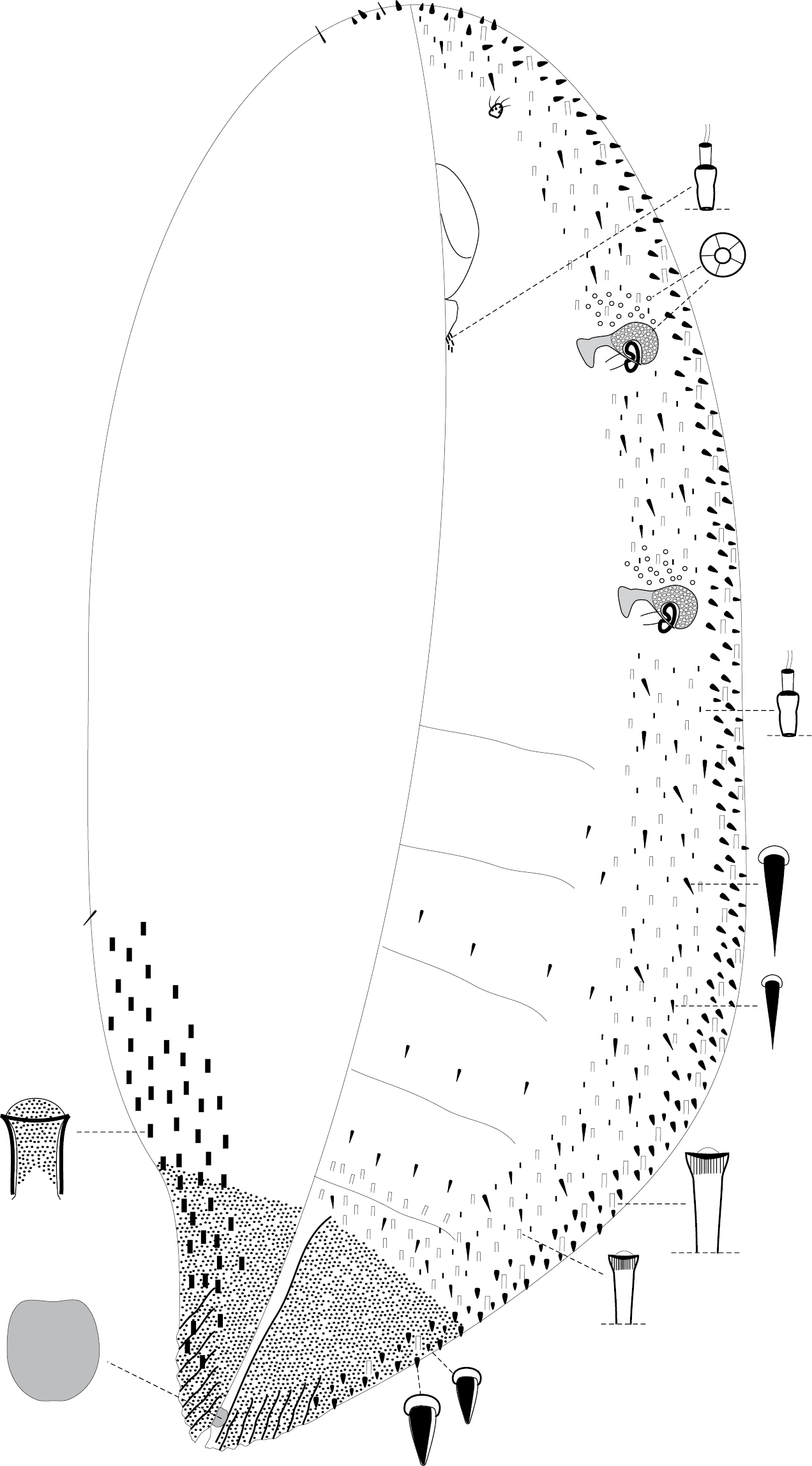
Fig. 1Body elongate oval, up to 7 mm long, slightly curved. Antennae small, 1-segmented, with several setae. Eyes and legs absent. Spiracles in two pairs; each with large and nearly circular and heavy sclerotized peritrema, covered by numerous quinquelocular pores. Posterior end of body heavily sclerotized on both surfaces even in very young females, abruptly narrowed and acutely pointed, ridged. Anal cleft short, about the same length as anal plate. Form of anal plate shown on the enlargement of Fig. 1. In general, the structure of anal complex is poorly visible because of heavy sclerotization of anal region of body, but it looks like anal complex in other species of the genus. Tubular ducts of 3 sizes: large tubular ducts about 18 µm long; medium-sized ducts about 10 µm long; and microtubular ducts about 7–8 µm long. All 3 types of ducts form ventral submarginal band as shown in Fig. 1. Microtubular ducts form also a group near labium. Quinquelocular pores form small groups near spiracles (with about 10–20 pores in each group). Dorsal invaginated setae (about 12–15 µm long) arranged along submarginal area of abdomen.
Aclerda pseudozoysiae sp. n., holotype.
The large and widely distributed genus Aclerda was comprehensively revised by
Holotype: female, K 884, vicinity of Jayapura, under the leaf sheath of undetermined grass (Poaceae), 1.XI. 2011, specimen in a black circle. Paratypes: 1 female on the same slide; 3 females on other slides and series of unmounted females and larvae in acet-ethanol; all with the same collecting data as holotype.
The species name “pseudozoysiae”is composed of pseudo (false) and “zoysiae”, and is intended to show its similarity to the related species, Aclerda zoysiae.
Both species are ovoviviparous; all stages of embryonic development occur inside the mother’s body. In view of the absence of any notes on ovisacs in other species of Aclerda in the coccidological literature, I suppose that the genus as a whole is ovoviviparous.
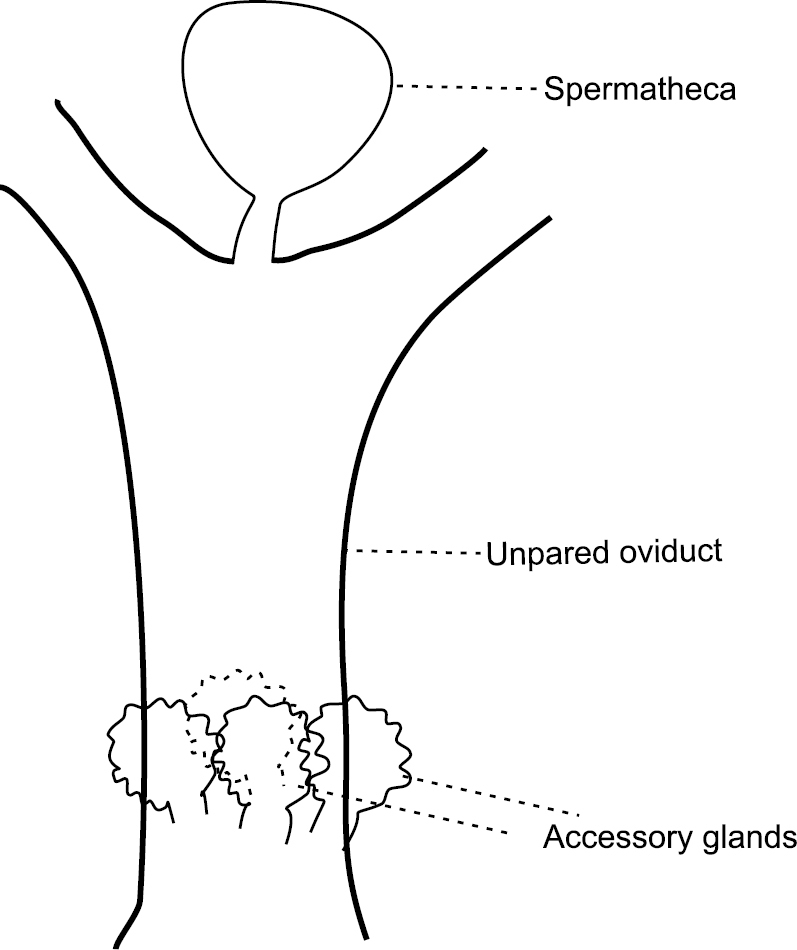
Both species have a spermatheca, attached medially between two lateral oviducts (Fig. 2).
Schematic drawing of oviducts and spermatheca of studied Aclerda spp.
Unexpectedly, the mode of reproduction is found to be absolutely different in these two species.
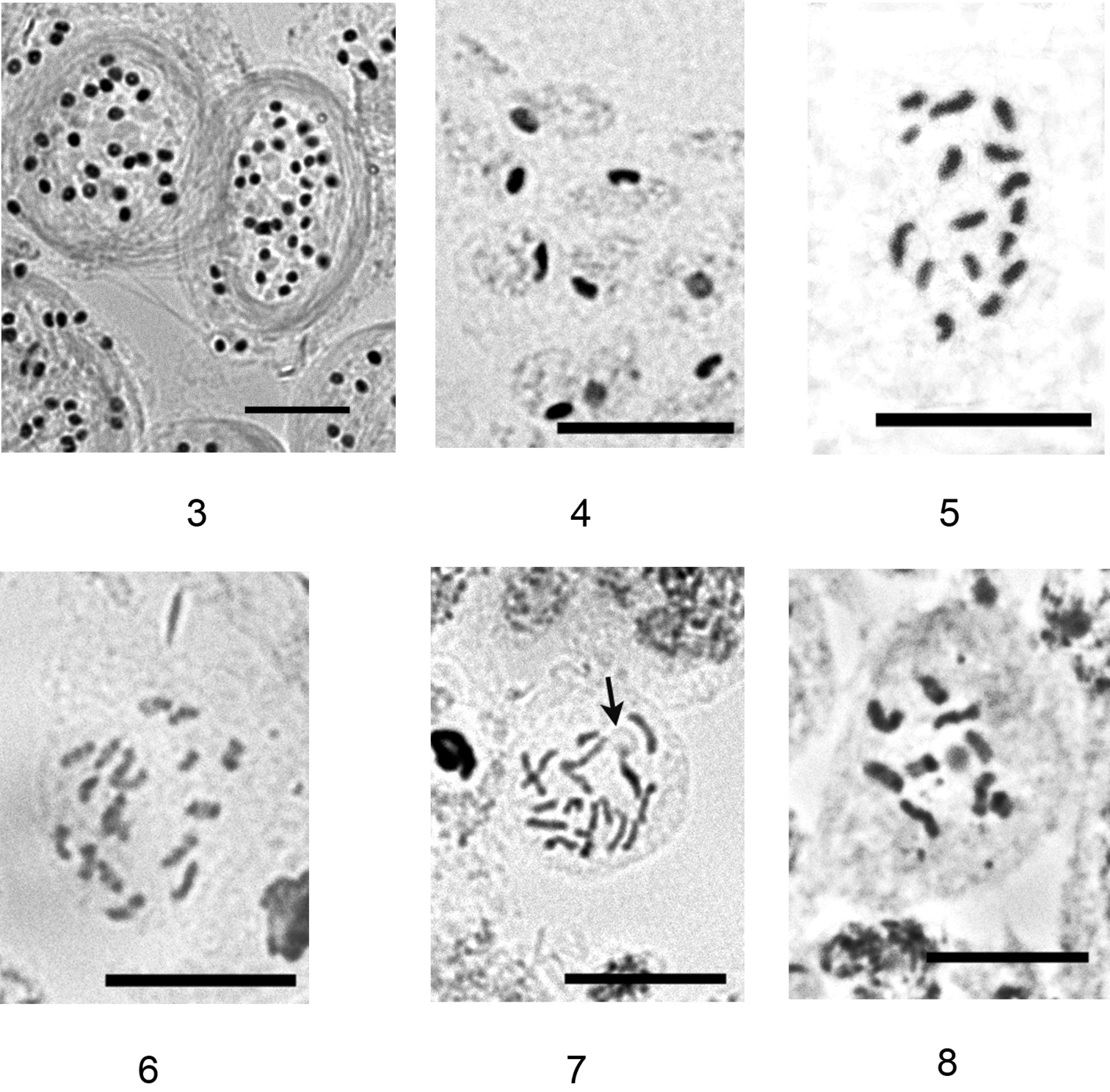
Aclerda pseudozoysiae has bisexual reproduction, with the presence of male stages of the life cycle in the analyzed population. The studied male ultimonymphs contained bundles of sperms in their testicles (Fig. 3). Specimens with meiotic divisions were not collected. Male larvae and nymphs, and about 50% of the embryos inside each of the four dissected adult females demonstrated a heterochromatinization of one haploid set of chromosomes (Fig. 4), that is common for the majority of cytogenetically studied groups of the superfamily Coccoidea (see, for example, the review of
On the contrary, in the studied population of Aclerda takahashii, no male stages of the life cycle were found and adult females did not have sperms and their spermathecae and oviducts. So, the species demonstrates a parthenogenetic form of reproduction. The diploid chromosomal number of Aclerda takahashii was found to be 18 (Fig. 6, 7) with chromosomes forming more or less gradual size series. Some of the cells showed a nucleolus located at the end of one of the longer chromosomes (Fig. 7) (the localization of NORs in scale insects was discussed earlier by
3–5 Aclerda pseudozoysiae sp. n.: 3 bundles of sperms, 4 heterochromatinization of one haploid set of chromosomes (black bodies inside the cells), 5 karyotype 6–8 Aclerda takahashii: 6 diploid karyotype, 7 diploid karyotype with nucleolus (arrowed) 8 haploid karyotype. Bar = 10 µm.
The work was partly supported by the RFBR grants 11-04-00734-a and 12-04-31797. The collection of Zoological Institute of Russian Academy of Sciences was financially supported by the Ministry of Education and Science of the Russian Federation (project no. 16.518.11.7070).