(C) 2012 Alexey A. Vakurin. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The karyotype of the Tsing-Ling (Huanghe) pika, Ochotona huangensis Matschie, 1908 from the forest habitats of the Qinling Mountains (Shaanxi Province, China) was described for the first time. The chromosome set contains 42 chromosomes (NFa=80). The autosomes are 15 meta-submetacentric pairs and 5 subtelocentric pairs. The X chromosome is a medium sized submetacentric; the Y chromosome is a small sized acrocentric. C-banding revealed a localization of heterochromatin in the pericentromeric regions of all autosomes.
Ochotona huangensis, pika, karyotype, chromosome, C-banding
The pikas Ochotona Link, 1795 are small (12–28 cm long) mammals of the order Lagomorpha Brandt, 1855. The developed sound signaling is a characteristic feature of most northern Ochotona species. They live either alone or in colonies, preferring taluses or open plains. The pikas find refuges in the crevices between rocks or dig burrows (
These animals occur in North America from Alaska to New Mexico. In the Old World pikas are distributed from the Arctic coast to the northern regions of Iran, Afghanistan, Pakistan, India and Burma, from the Polar Urals in the West to the Pacific coast in the East, including Chukotka, Kamchatka peninsula, Hokkaido Island and also in territory of North Korea (
The pikas are one of the most ancient groups of the placental mammals (
The majority of modern taxonomists recognize 30 species of pikas and they divide them into three subgenera: Pika Lacepede, 1799, Ochotona Link, 1795 and Conothoa Lyon, 1904 (
Subgenera system of the genus Ochotona and variability of the diploid chromosome number (2n). NF – the fundamental number of chromosomal arms.
| Subgenus | Species | 2n | NF | Banding methods | References |
|---|---|---|---|---|---|
| Pika | Ochotona argentata Howell, 1928 | 38 | 76 | C, NOR |
|
| Ochotona hoffmanni Formozov et al., 1996 | 38 | 76 | G, C |
|
|
| Ochotona pallasi (=pricei) Gray, 1867 | 38 | – | – | ||
| 76 | G, C |
|
|||
| Ochotona hyperborea Pallas, 1811 | 40 | – | – |
|
|
| – | – |
|
|||
| 76 | C |
|
|||
| Ochotona alpina Pallas, 1773 | 42 | – | – |
|
|
| 78 | G, C |
|
|||
| – | – |
|
|||
| Ochotona collaris Nelson, 1893 | 68 | 90 | – |
|
|
| Ochotona princeps Richardson, 1828 | 68 | 86 | – |
|
|
| G, C |
|
||||
| Ochotona | Ochotona huangensis Matschie, 1908 | 42 | 84 | C | Our data |
| Ochotona curzoniae Hodgson, 1858 | 46 | 68 | G, C |
|
|
| Ochotona nubrica Thomas, 1922 | 48 | – | – | Formozov et al. (personal communication) | |
| Ochotona dauurica Pallas, 1776 | 50 | – | – |
|
|
| 72 | G, C |
|
|||
| Ochotona pusilla Pallas, 1769 | 68 | – | – |
|
|
| 106 | G, C |
|
|||
| Conothoa | Ochotona forresti Thomas, 1923 | 54 | – | DAPI |
|
| Ochotona rufescens Gray, 1842 | 60 | 86 | – |
|
|
| – | – |
|
|||
| – | G, C |
|
|||
| 90 | G, C |
|
|||
| Ochotona roylei Ogilby, 1839 | 62 | – | G, NOR |
|
|
| Ochotona macrotis Gunther, 1875 | 62 | 86 | – |
|
|
| Ochotona rutila Severtsov, 1873 | 62 | – | – |
|
|
| 86 | G, C |
|
|||
| Ochotona ladacensis Gunther, 1875 | 68 | – | – | Formozov et al. (personal communication) |
Up to 24 species of pika inhabit China (
During the last four decades, the systematics of the northern Palearctic and North American pikas has been well developed, but the system of subgenera and superspecies groups was periodically reconsidered with increase of number of morphological, morpho-ecological features and descriptions of karyotypes (
In this paper the karyotype of Ochotona huangensis Matschie, 1908 is described for the first time. This species has a few synonyms of common names: Tsing-Ling pika, Huanghe pika, Qinling pika. We will use the common name as Tsing-Ling pika, before conducting the full revision of this species. We adhered to intrageneric taxonomy proposed by
One male of Ochotona huangensis was used as a material for this study. It was caught on Sept. 12, 2005 during the joint Russian-Chinese expedition to the Qinling Mountains near the Foping village of Shaanxi Province, China. The pika was caught on a glade of the pine-oak forest, at height less than 1800 m (33°28'36, 3"N, 108°30'18, 6"E). This was slightly below the typical habitat for the Tsing-Ling (Huanghe) pika: a birch-fir forest located above 2000 m (
Identification of the pika from the Qinling Mountains was performed by morphological characters. We used a molecular express analysis of the cytochrome b gene of mtDNA for confirming of taxonomic status of this specimen to the species Ochotona huangensis. Total genomic DNA was extracted from liver tissue by standard protocol (
L14075och 5’ – gta tgt cat aat tct tac atg ga – 3’
H15374och 5’ – gta agc cga ggg cgt ctt tg – 3’
The primers were designed according to published whole mitochondrial sequence of pika Ochotona collaris (GenBank NCBI (www.ncbi.nlm.nih.gov ) № NC_003033). The PCR program consisted of 94 °C for 5 min followed by 35 cycles at 94 °C for 1 min, 62 °C for 1 min, and 72 °C for 3 min. A final amplification step completed the PCR at 72 °C for 7 min.
The PCR products were purified by Sin Column PCR Product Purification Kit (Evrogen, Moscow, Russia). The directly sequencing of the purified PCR products was performed using ABI PRISM BigDyeTM Terminator v3.1 (Applied Biosystems, Inc., Foster City, California) with an automatic DNA sequencer (Model ABI PRISM 3100-Avant Genetic Analyzer; Applied Biosystems, Inc., Foster City, California). The same primers were used for sequencing PCR from both directions.
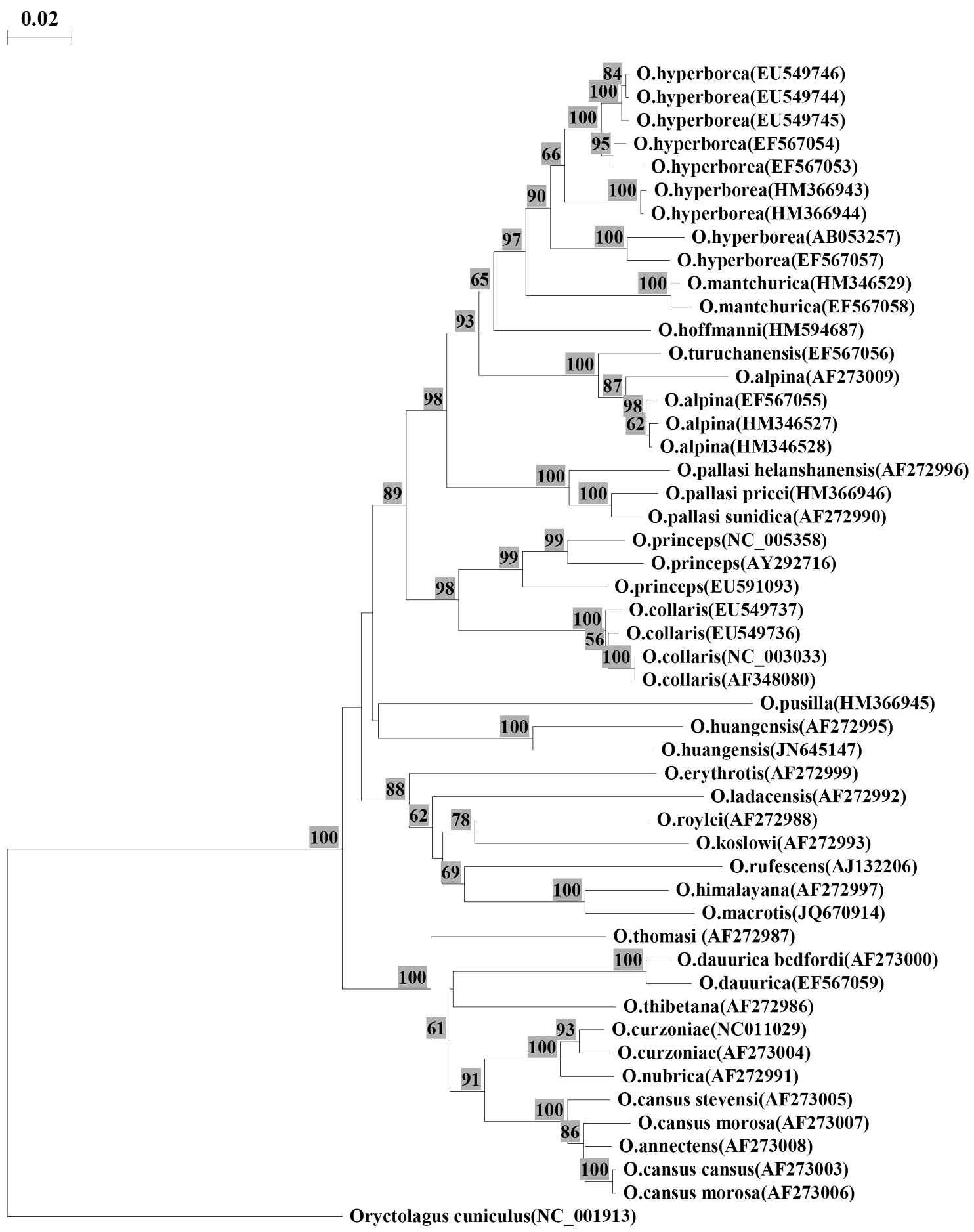
The obtained sequence (GenBank NCBI № JN645147) was compared with full-length cytochrome b (1140bp) of 23 pikas species published by different authors in GenBank. The alignment of sequences was conducted by the program BIOEDIT v7.0.9 (Tom Hall, Ibis Biosciences). The genetic distances were estimated with neighbor-joining method, using Kimura two-parameter model. The tree was constructed by including all transitions and transversions with TREECON v3.1b (Yves Van De Peer, Germany). A rabbit Oryctolagus cuniculus Linnaeus, 1758 was selected as an outgroup (Fig. 1).
Neighbor-joining distance tree constructed using the Kimura two-parameter model for complete sequence cytochrome b (1140 bp). Numbers on branches indicate bootstrap support; values less than 50 are not shown. Numbers following the species names indicate the GenBank accession numbers.
Method of cell division stimulation in the red bone marrow with baker’s yeast solution was used for preparation of chromosomal slides (
The chromosomal slides were analyzed on light microscope AxioSkop 40 with lens x100. Photographs were performed with the digital camera AxioCamHR using the program AXIOVISION 4.7 (Carl Zeiss MicroImaging GmbH, Germany). The morphology of the chromosomes was assessed visually without measurements (
Independence ofthe Ochotona huangensis taxon was suggested by molecular studies (
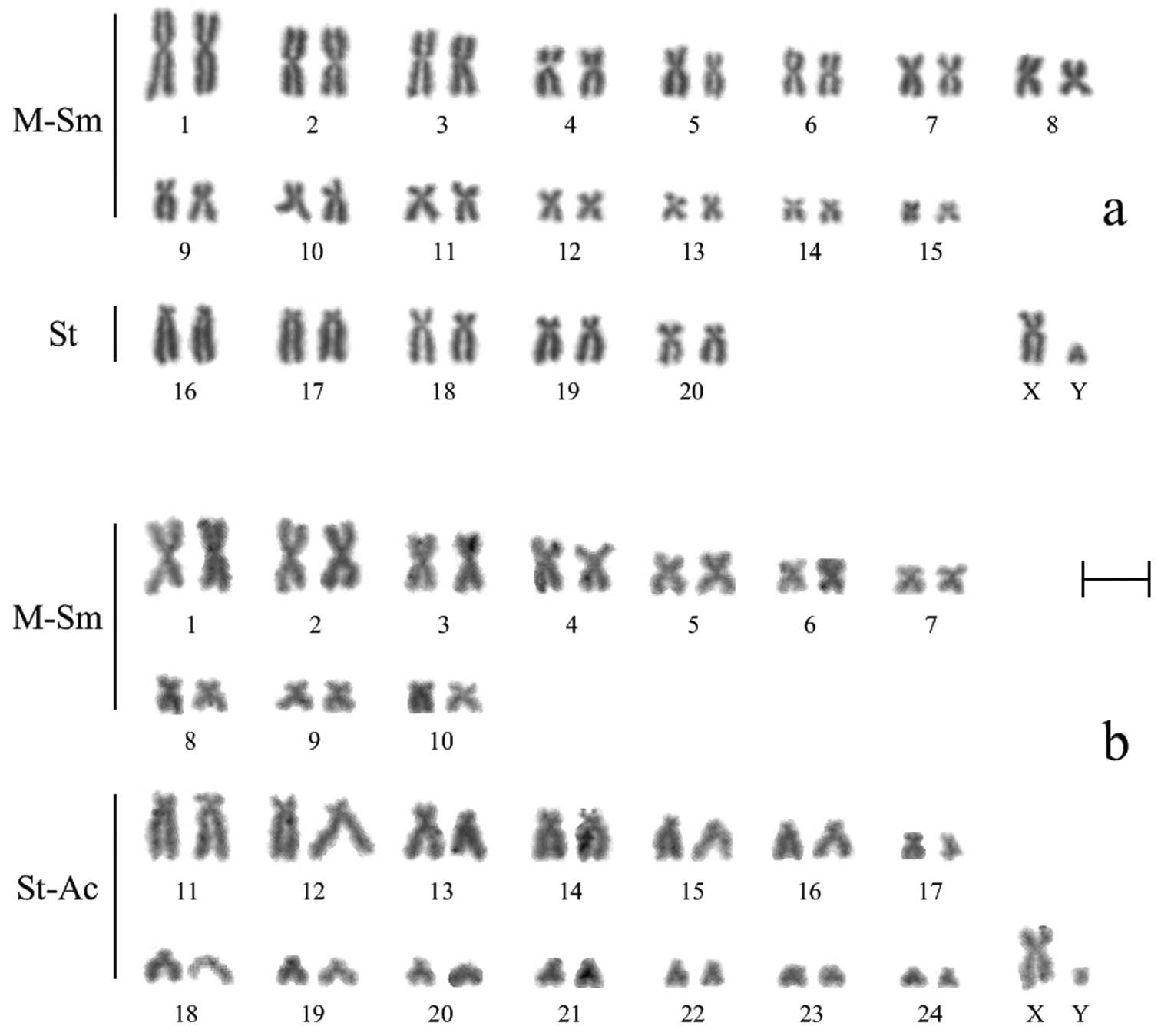
According to the results of counting on 40 metaphase plates, the diploid chromosome number of Ochotona huangensis is 42 (NFa=80). Morphologically two groups of autosomes were identified. The first group consists of 15 pairs (3 large, 8 medium and 4 small) meta-submetacentric chromosomes. The second group consists of 5 pairs rather large, gradually decreasing in size, subtelocentric chromosomes. The X chromosome is a medium sized submetacentric, the Y chromosome is a small acrocentric (Fig. 2a).
Routine stained karyotypes of Ochotona huangensis (a) and Ochotona dauurica (b): M-Sm meta-submetacentric chromosomes, St subtelocentric chromosomes, St-Ac subtelo- and acrocentric chromosomes. Bar = 5 μm.
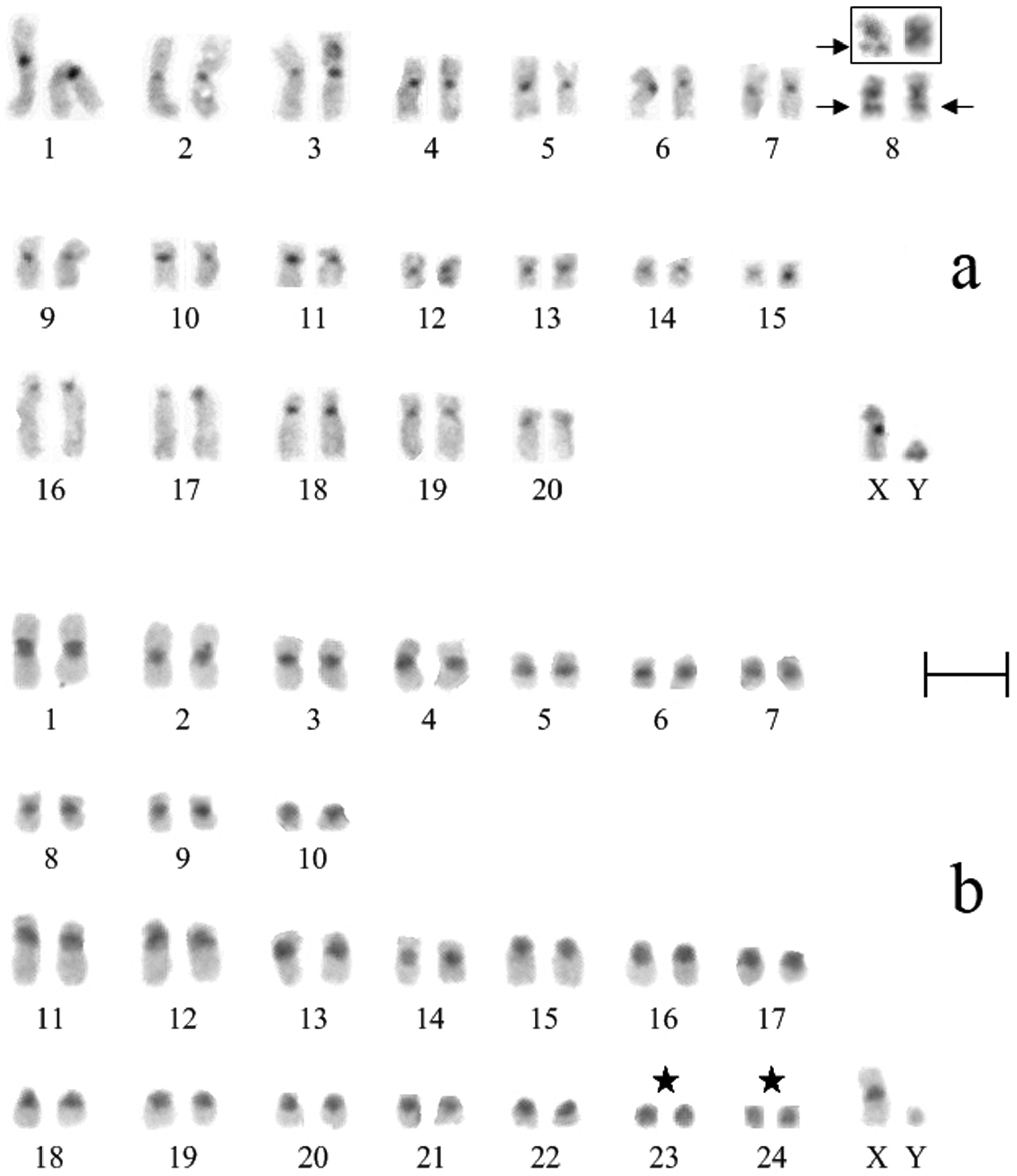
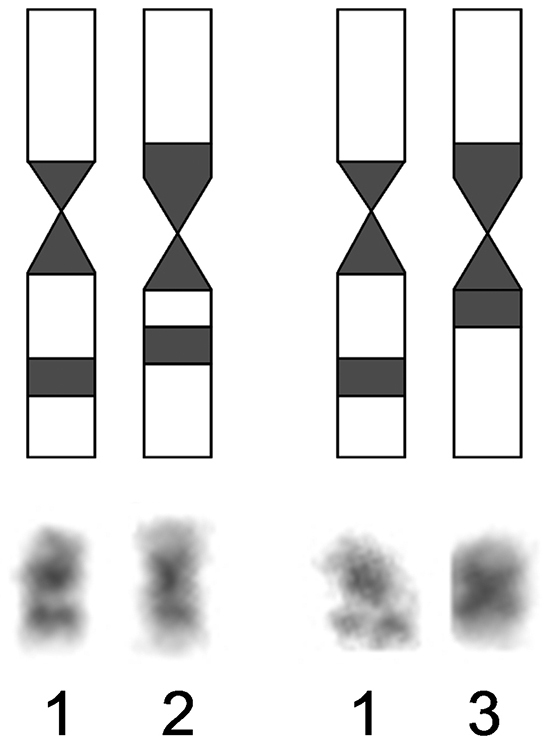
Nineteen metaphase cells stained for the structural heterochromatin (C-banding) were analyzed. The clearly stained pericentromeric heterochromatic blocks, which sizes were approximately the same, were identified at all chromosomes of Ochotona huangensis. The heteromorphism was detected by localization of heterochromatic blocks on the 8-th pair of autosomes. An intercalary heterochromatic block was always detected in the long arm of one homologue of the 8-th pair. Also, that homologue had the pericentromeric block of heterochromatin. In the second homologue of this pair, the intercalary heterochromatic block was detected in nine metaphase cells. In the remaining cells, only the larger pericentromeric heterochromatic block was detected in this homologue. By that, the euchromatic site, which separates the intercalary heterochromatic block, was broader on the first homologue than that on the second homologue (Fig. 4). We can’t characterize this phenomenon in details and discuss about its nature, because of the absence of sufficient material. So we leave it only as an observed fact. The X chromosome has a pericentromeric block of heterochromatin. The heterochromatic region occupies 2/3 of the lower arm on the Y chromosome (Fig. 3a).
The Daurian pika, which like Ochotona huangensis belongs to the subgenus Ochotona, was studied for a comparative karyotype analysis. The karyotype of Ochotona dauurica contains 50 chromosomes (NFa=68) which are grouped in 10 meta-submetacentric pairs (3 large, 2 medium and 5 small) and 14 subtelo- and acrocentric pairs of autosomes. The X chromosome is a submetacentric, similar in size to the 3-rd or 4-th pairs of autosomes, the Y chromosome is a very small acrocentric (Fig. 2b). The karyotype of the Daurian pika does not differ from that which was previously described in the literature (
Analysis of 15 C-stained metaphase plates showed that all autosomes of Ochotona dauurica have the large pericentromeric heterochromatic blocks which were intensively stained. The 10-th and 15-th – 22-th pairs of autosomes have completely heterochromatic short arms. The last two small pairs of autosomes (23-th and 24-th) are composed of heterochromatin entirely. The large pericentromeric block of the X chromosome occupies 1/3 of the long arm. Heterochromatic structure of the Y chromosome was not confirmed (Fig. 3b) compared with published data (
C-banded karyotypes of Ochotona huangensis (a) and Ochotona dauurica (b): ★ – intercalary heterochromatic blocks, → – autosomes entirely consisted of heterochromatin. Bar = 5 μm.
Scheme of localization of heterochromatic blocks on 8-th pair of Ochotona huangensis: 1 – the first homologue, 2 and 3 – the second homologue in two variants.
An obvious resemblance between the karyotypes of Ochotona huangensis and Ochotona dauurica was seen by the routine staining, despite of some bigger size of the first pair of Ochotona huangensis. The first four meta-submetacentric pairs of Ochotona dauurica are similar to the 2nd – 5-th pairs of Ochotona huangensis autosomes. The remaining five meta-submetacentric pairs of Ochotona dauurica, except the 10-th pair, are similar to the last five pairs of the first group of Ochotona huangensis autosomes. The 11-th – 15-th autosomes of Ochotona dauurica are very similar to the second subtelocentric group of Ochotona huangensis by morphology and sizes, with a loss of the little part of the upper arm on the 20-th pair. The absence of G-stained chromosomes not allows us to do unambiguous conclusion about the relationship between the karyotypes of Ochotona dauurica and Ochotona huangensis. Such species as Ochotona alpina (2n=42), Ochotona hyperborea (2n=40), Ochotona pallasi (2n=38) and Ochotona argentata (2n=38) of the subgenus Pika (
The C-banding patterns of Ochotona dauurica specimens from Transbaikalia (near the station Armagotuy) and Mongolia (Selenge aimag, near Shamar) (
A tendency of heterochromatin decreasing is confirmed in row of pikas: from species with a large number of chromosomes to species with a smaller number, while comparing the overall C-banding pattern of Ochotona dauurica and Ochotona huangensis (
The species Ochotona alpina (subgenus Pika) is similar to Ochotona huangensis by the diploid chromosome number, but it has another arrangement of heterochromatin. Pericentromeric heterochromatin is detected only on 6 submetacentric and 5 subtelocentric pairs of Ochotona alpina autosomes (
The molecular studies of the genus Ochotona (
The position of Ochotona huangensis is ambiguous in this system. According to the data of study of the cytochrome b and the ND4 gene (
The authors express their sincere thanks to Dr A. P. Kryukov for establishing Russian-Chinese collaboration and attending the expedition to the Qinling, Dr M. V. Pavlenko for help in collecting of material, Dr I. V. Kartavtseva for valuable comments, advice and help in writing the manuscript. Special thanks to Dr N. A. Formozov for his help in molecular identifying of the Ochotona huangensis species.
This work was partially supported by Russian Foundation for Basic Research, Project no. 06-04-39015; Far East Branch Russian Acad. Sci., Project no. 09-II-CO-06-007 and grant “Complex expeditionary researches of environment of Amur river basin 2004-2007”. The study was conducted with the technical support of laboratory microscopy of center for collective use “Biotechnology and Genetic Engineering” (Establishment of IBSS FEB RAS).