(C) 2013 Roberto Laridondo Lui. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Auchenipteridae is divided in two subfamilies, Centromochlinae and Auchenipterinae. Centromochlinae has 31 valid species, from which 13 are included in the genus Tatia Miranda Ribeiro, 1911. Among these, Tatia jaracatia Pavanelli & Bifi, 2009 and Tatia neivai (Ihering, 1930) are the only two representative species from the Paraná-Paraguay basins. This study aimed to analyze cytogenetically these two species and thus provide the first chromosomal data for the genus. Although Tatia jaracatia and Tatia neivai presented 2n=58 chromosomes, some differences were observed in the karyotypic formula. The heterochromatin was dispersed in the centromeric and terminal regions of most chromosomes of Tatia jaracatia, and only in the terminal region of most chromosomes of Tatia neivai. The AgNORs were detected in the subtelocentric pair 28 for both species, which was confirmed by FISH with 18S rDNA probe. The 5S rDNA sites were detected in four chromosome pairs in Tatia jaracatia and three chromosome pairs in Tatia neivai. Both species of Tatia presented great chromosomal similarities among themselves; however, when compared to other species of Auchenipteridae, it was possible to identify some differences in the karyotype macrostructure, in the heterochromatin distribution pattern and in the number and position of 5S rDNA sites, which until now seems to be intrinsic to the genus Tatia.
Pericentric inversions, NORs, C-banding, 5S rDNA-FISH, 18S rDNA-FISH
Among the Siluriformes, Auchenipteridae comprises a fish group endemic to the Neotropical region. The family comprises 20 genera and about 90 species (
The genus Tatia is found in the eastern region of the Andes, with wide distribution in South American drainages (
Chromosomal analyses in Auchenipteridae are scarce and restricted to few species of the genera Ageneiosus La Cepède, 1803, Auchenipterus Bleeker, 1862, Glanidium and Parauchenipterus (Linnaeus, 1766). The two analyzed Ageneiosus species demonstrate diploid number of 56 chromosomes (
Chromosomal analysis was performed on 17 specimens (15 males and 2 females) of Tatia neivai from Machado River, a tributary of the Bugres River, Paraguay River basin, Denise city, Mato Grosso, Brazil (14° 40'43"S, 57°00'47"W), and 10 specimens (7 males and 3 females) of Tatia jaracatia from the Iguaçu River basin, Capanema city, Paraná, Brazil (25°35'19"S, 53°54'48"W). The specimens were deposited in the fish collection of Museum of Zoology of University of São Paulo (Tatia jaracatia, MZUSP 109792; Tatia neivai, MZUSP 109794).
Specimens were previously treated with 0.05% colchicine solution (1 ml/100 g body weight), 30-40 minutes before sacrifice, and the cell suspension of mitotic chromosomes was obtained from the anterior kidney cells (
The fluorescence in situ hybridization (FISH) was performed according to
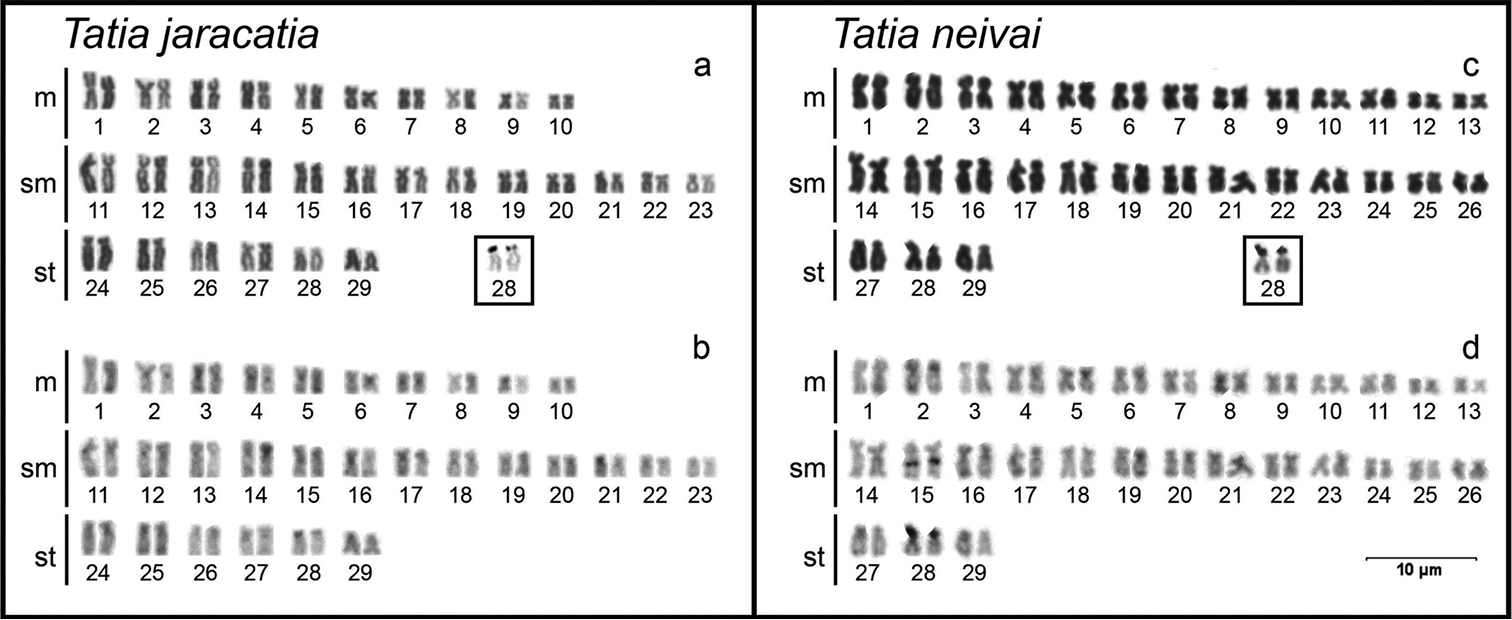
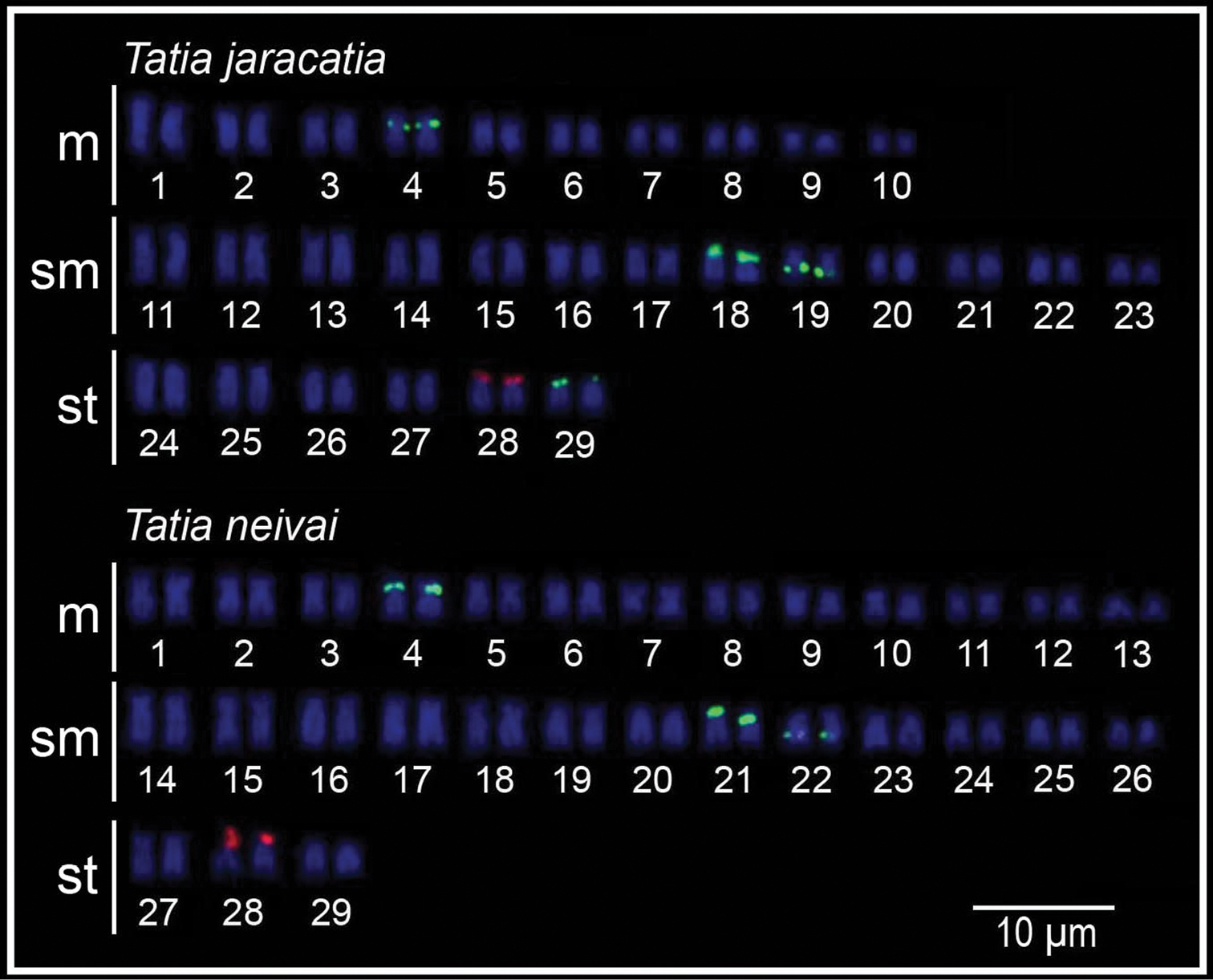
Cytogenetical analysis revealed the diploid number of 58 chromosomes (20m+26sm+12st, FN=116) (Fig. 1a). The heterochromatin presented itself disperses in the centromeric and terminal regions of most chromosomes of the karyotype (Fig. 1b). The silver nitrate impregnation showed only the subtelocentric pair 28 marked in the terminal position of the short arm (Fig. 1a, in box). FISH with 18S rDNA probe showed only one labeled chromosome pair (pair 28) corresponding to the silver nitrate impregnation. The 5S rDNA sites were detected in 4 chromosome pairs (pairs 4, 18, 19 and 29), on the short arm in interstitial position of the metacentric pair 4, on the short arm in terminal position of the submetacentric pairs 18 and 29, and on the long arm in interstitial position of the submetacentric pair 19 (Fig. 2).
Karyotypes of Tatia jaracatia (a, b) and Tatia neivai (c, d) stained with Giemsa (a, c) and sequentially C-banded (b, d). The AgNORs bearing chromosomal pair is presented in box.
Cytogenetical analysis revealed the diploid number of 58 chromosomes (26m+26sm+6st, FN=116) (Fig. 1c). The heterochromatin showed itself poorly marked and dispersed in the terminal region of most chromosomes of the karyotype, with the exception of two conspicuous blocks: one in interstitial position on the long arm of submetacentric pair 15, and other in terminal position on the short arm of subtelocentric pair 28 (Fig. 1d), corresponding to the NORs (Fig. 1c, in box). FISH with 18S rDNA probe showed only one labeled chromosome pair, the subtelocentric pair 28, corresponding with the silver nitrate impregnation. The 5S rDNA sites were detected in 3 chromosome pairs (pairs 4, 21 and 22), being in the interstitial position of the short arm of metacentric pair 4, in terminal position of the short arm of submetacentric pair 21, and in interstitial position of the long arm of submetacentric pair 22 (Fig. 2).
No intraspecific polymorphism related to diploid number, karyotypic formula, C banding, 5S and 18S rDNA (including AgNORs) were observed in both species.
Karyotypes of Tatia jaracatia and Tatia neivai 5S rDNA-FISH (FITC, green) and 18S rDNA-FISH (digoxigenin, red).
Chromosomal studies in Auchenipteridae have shown that most analyzed species have diploid number of 58 chromosomes (
The fundamental number (FN=116) found for the two Tatia species in this paper is higher than found in other Auchenipteridae species studied so far. This difference is due to an increase in the number of chromosomes bearing two arms in the detriment of chromosomes carrying only one arm (Fig. 1a, c). This absence of acrocentric chromosomes was not detected in other species of the family yet, and seems to be an intrinsic characteristic of the genus Tatia, or at least of a specific clade formed by the species studied here. Thus, considering the maintenance of the diploid number, the variations in the karyotypic formula and FN of analyzed species, when compared with other species from others Auchenipteridae genus, it is evident that non-Robertsonian rearrangements, here represented by pericentric inversions, must be active mechanisms in the karyotypic diversification of Tatia species.
The heterochromatin distribution pattern found in Tatia jaracatia and Tatia neivai differs in some aspects from other Auchenipteridae species. Besides heterochromatic blocks in the terminal region of chromosomes, which are commonly found in most Auchenipteridae species, heterochromatin sites were observed in the centromeric region of some chromosomes in Tatia jaracatia, and a conspicuous block in the interstitial region of the submetacentric pair 15 of Tatia neivai (Fig. 1b, d). No interstitial heterochromatin blocks were detected in Tatia jaracatia.
The silver nitrate impregnation had only one subtelocentric chromosome pair marked on the short arm in terminal position (pair 28) in both species (Fig. 1, in box), as confirmed by FISH with 18S rDNA probe (Fig. 2). This pair is likely correspondent between species. According to
The data of 5S rDNA sites physical mapping by FISH in Auchenipteridae are scarce and only refer to Parauchenipterus galeatus populations (
According to the phylogeny of
The authors are grateful to Dr. Heraldo Antonio Britski for the identification of the specimens; the laboratory technician Pedro Luis Gallo and the GETECH (Grupo de Pesquisa em Tecnologia de Produção e Conservação de Recursos Pesqueiros e Hídricos of UNIOESTE) for assistance with the samplings; the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for the authorization for the material collection. This study was financed by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).