(C) 2013 Robert B. Angus. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An account is given of the karyotypes of 29 species of medium sized Dytiscidae (Coleoptera). Of the 20 species of Agabus Leach, 1817, 18 have karyotypes comprising 21 pairs of autosomes and sex chromosomes which are either X0(♂) or XX (♀). These species are Agabus serricornis (Paykull, 1799), Agabus labiatus (Brahm, 1791), Agabus congener (Thunberg, 1794), Agabus lapponicus (Thomson, 1867), Agabus thomsoni (J. Sahlberg, 1871), Agabus confinis (Gyllenhal, 1808), Agabus sturmii (Gyllenhal, 1808), Agabus bipustulatus (Linnaeus, 1767), Agabus nevadensis Håkan Lindberg, 1939, Agabus wollastoni Sharp, 1882, Agabus melanarius Aubé, 1837, Agabus biguttatus (Olivier, 1795), Agabus binotatus Aubé, 1837, Agabus affinis (Paykull, 1798), Agabus unguicularis (Thomson, 1867), Agabus ramblae Millan & Ribera, 2001, Agabus conspersus (Marsham, 1802) and Agabus nebulosus (Forster, 1771). However two species, Agabus infuscatus Aubé, 1838 and Agabus adpressus Aubé, 1837, have developed a neo-XY system, with karyotypes comprising 21 pairs of autosomes and XY sex chromosomes (♂). No chromosomal differences have been detected between typical Agabus bipustulatus and Agabus bipustulatus var. solieri Aubé, 1837, nor have any been found between the three species of the Agabus bipustulatus complex (Agabus bipustulatus, Agabus nevadensis and Agabus wollastoni). The four species of Colymbetes Clairville, 1806, Colymbetes fuscus (Linnaeus, 1758), Colymbetes paykulli Erichson, 1837, Colymbetes piceus Klug, 1834 and Colymbetes striatus (Linnaeus, 1758) have karyotypes comprising 20 pairs of autosomes and sex chromosomes which are X0 (♂), XX (♀). Two of the species of Rhantus Dejean, 1833, Rhantus exsoletus (Forster, 1771) and Rhantus suturellus (Harris, 1828) have karyotypes comprising 20 pairs of autosomes and X0/XX sex chromosomes, but the other three species, Rhantus grapii (Gyllenhal, 1808), Rhantus frontalis (Marsham, 1802) and Rhantus suturalis (Macleay, 1825) have 22 pairs of autosomes and X0/XX sex chromosomes. Agabus congener and Rhantus suturellus may have one B-chromosome. Nine of the species have previously published karyotype data but for seven of these the data are wrong and are here corrected.
Chromosomes, karyotypes, sex chromosome systems, Dytiscidae, Agabus, Colymbetes, Rhantus
When
The present paper reports on 20 Agabus species, of which only four had previously published chromosome data (wrong for three of the species), 4 Colymbetes, all of which have previously published data, though for three of the species these data were wrong, and 5 Rhantus of which one species had published data, again wrong. This gives a net increase to over 100 in the number of dytiscid species for which information on chromosome numbers are available. The data have been gathered over more than 25 years, and include the results of research projects by three undergraduate students of Royal Holloway, University of London, supervised by R. B. Angus. D. E. Wenczek (1994) studied Rhantus Dejean, J. C. Carter (2001) Rhantus and Colymbetes, and M. J. Clery (2009) made a special study of the Agabus bipustulatus (Linnaeus) species group.
The species studied, with their localities of origin, collectors and dates, as well as the number of specimens yielding successful preparations, is given in Table 1. Nomenclature and classification follow
Preparations were made from adult beetles, using mid-gut, testis and ovary, following the protocol given by
Material studied.
| Species | Locality | Collector, date | Material |
|---|---|---|---|
| Genus Agabus Leach, 1817 | |||
| Subgenus Agabus s. str. | |||
| Agabus serricornis (Paykull, 1799) | SWEDEN: Västerbotten, Åmsele. | A. N. Nilsson, 1990 | 1♂ |
| Agabus labiatus (Brahm, 1791) | FINLAND: Lapponia Inarensis, Inari | R. B. Angus, 2008 | 1♂, 1♀ |
| Subgenus Acatodes C. G. Thomson, 1859 | |||
| Agabus congener (Thunberg, 1794) | SCOTLAND: Ayrshire, Knockewart Moss | G. N. Foster, 1986 | 2♂♂, 1♀ |
| SWEDEN: Västerbotten, Sirapsbaken | A. N. Nilsson, 1986 | 2♂♂, 1♀ | |
| Agabus lapponicus (Thomson, 1867) | SWEDEN: Västerbotten, Skörträskberget | A. N. Nilsson, 1986 | 3♂♂, 1♀ |
| Agabus thomsoni (J. Sahlberg, 1871) |
NORWAY: Finnmark east, Bugøynes | R. B. Angus, 2008 | 1♂ |
| Agabus confinis (Gyllenhal, 1808) | SWEDEN: Västerbotten, Vindeln, Strycksele | A. N. Nilsson, 1991 | 3♀♀ |
| Agabus sturmii (Gyllenhal, 1808) | ENGLAND: Surrey, Chobham Common | R. B. Angus, 1991 | 1♂, 1♀ |
| Agabus infuscatus Aubé, 1838 | NORWAY: Finnmark east, Bugøynes | R. B. Angus, 2008 | 1 ♂ |
| Subgenus Gaurodytes C. G. Thomson, 1859 | |||
| Agabus bipustulatus (Linnaeus, 1767) | ENGLAND: Surrey, Wisley Common | R. B. Angus & M. J. Clery, 2008 | 3♂♂ |
| Hampshire, Woolmer Bog | R. B. Angus, 2008 | 3♂♂, 1 ♀ | |
| Worcestershire, Wyre Forest | R. B. Angus & M. J. Clery, 2008 | 3 ♂♂ | |
| FINLAND: Lapponia Inarensis, Inari | R. B. Angus, 2008 | 1 ♂ | |
| SWEDEN: Norbotten, near Umeå | M. Drotz, 1996 | 1 ♂ | |
| Agabus bipustulatus var. solieri Aubé, 1837 | SWITZERLAND, Valais, small lake S of Illsee | R. B. Angus, 2008 | 3♂♂, 1 ♀ |
| Valais, ditch near the Moiry glacier | R. B. Angus, 2008 | 2 ♂♂ | |
| FRANCE: Hautes-Alpes, Guillestre | M. Drotz, 1998 | 2♂♂ | |
| Agabus nevadensis Håkan Lindberg, 1939 | SPAIN: Granada, Sierra Nevada | M. Drotz, 1999 | 1 ♂, 1 ♀ |
| Agabus wollastoni Sharp, 1882 | MADEIRA: Pico Arieño | A. N. Nilsson, 1998 | 2 ♂♂, 1 ♀ |
| Agabus melanarius Aubé, 1837 | ENGLAND: East Sussex, Hindleap Warren | R. B. Angus & M. J. Clery, 2008 | 1 ♂, 1 ♀ |
| Agabus biguttatus (Olivier, 1795) | EGYPT (Saleh Ahmed et al., 2000): El Noqra | R. Saleh Ahmed & R. B. Angus, 1994 | 1 ♂ |
| SARDINIA: Medio Campidano, Giara di Gesturi | R. B. Angus, 1994 | 1♂ | |
| Agabus binotatus Aubé, 1837 | CORSICA: Corse-du-Sud, Col de Vizzavona. | R. B. Angus, 1993 | 1 ♂ |
| Agabus affinis (Paykull, 1798) | ENGLAND: Hampshire, New Forest | R. B. Angus, 1987 | 1 ♂ |
| Agabus unguicularis (Thomson, 1867) | ENGLAND: Norfolk, East Walton Common | R. B. Angus, 1987 | 2 ♂♂ |
| Agabus ramblae Millan & Ribera, 2001 | SPAIN: Huesca, Villanueva de Sigena, Barranco del Hospital | I. Ribera, G.N. Foster, D. Lott & P. Aguilera, 1995 | 2♂♂ |
| Murcia, Rambla de Majada en El Pilón | A. Millan, 1995 | 1 ♀ | |
| Agabus conspersus (Marsham, 1802) | ENGLAND: Hampshire, Keyhaven | R. B. Angus, 1993 | 1 ♂ |
| Agabus nebulosus (Forster, 1771) | ENGLAND: East Sussex, Cuckmere Haven | R. B. Angus, 1993 | 1♂ |
| CANARY ISLANDS: Tenerife | A. N. Nilsson, 1994 | 1♂, 2 ♀♀ | |
| Agabus adpressus Aubé, 1837 | NORWAY: Finnmark east, Bugøynes | R.B. Angus, 2008 | 1 ♂ |
| Genus Colymbetes Clairville, 1806 | |||
| Colymbetes fuscus (Linnaeus, 1758) | ENGLAND: Surrey, Wisley Common | R. B. Angus, 2000 | 1 ♂ |
| FRANCE: Indre, Pinail | R. B. Angus, 2000 | 1 ♂ | |
| Colymbetes paykulli Erichson, 1837 | SWEDEN: Ångermanland, Hörnsjö, lake Uthörnsjön | A. N. Nilsson, 2000 | 1 ♂ |
| Ångermanland, Mullsjö | A. N. Nilsson, 2000 | 1 ♂ | |
| Colymbetes piceus Klug, 1834 | Egypt (Saleh Ahmed et al., 2000): El Noqra | R. Saleh Ahmed & R. B. Angus, 1994 | 1 ♂ |
| Colymbetes striatus (Linnaeus, 1758) | SWEDEN: Ångermanland, Hörnsjö, lake Uthörnsjön | A. N. Nilsson, 2000 | 1 ♂ |
| Genus Rhantus Dejean, 1833 | |||
| Subgenus Nartus Zaitsev, 1907 | |||
| Rhantus grapii (Gyllenhal, 1808) | ENGLAND: Dorset, Studland Heath | R. B. Angus, 1993 | 2♂♂ |
| Subgenus Rhantus s. str. | |||
| Rhantus exsoletus (Forster, 1771) | ENGLAND: Dorset, Studland Heath | R. B. Angus, 1993 | 1 ♂ |
| Norfolk, Gayton Thorpe Common | R. B. Angus, 1993 | 1 ♂ | |
| Rhantus frontalis (Marsham, 1802) | ENGLAND: Norfolk, Gayton Thorpe Common | R. B. Angus, 1993 | 1 ♂ |
| Norfolk, Thompson Common | R. B. Angus, 1993 | 1 ♂ | |
| Rhantus suturalis (Macleay, 1825) | ENGLAND: Dorset, Studland Heath | R. B. Angus, 2000 | 1 ♂ |
| Middlesex, Staines Moor | R. B. Angus, 2000 | 1 ♂ | |
| KUWAIT: Ras Az Zawr | R. B. Angus, 1996 | 1 ♂ | |
| Rhantus suturellus (Harris, 1828) | FRANCE: Indre, Pinail | R. B. Angus, 2000 | 1 ♂ |
| ENGLAND: Dorset, Studland Heath | R. B. Angus, 1993, 2000 | 1♂, 2 ♀♀ | |
Chromosome measurements were made on screen and were used for calculating Relative Chromosome Length (RCL), the length of each chromosome expressed as a percentage of the total haploid autosome length in the nucleus. This compensates for differing degrees of chromosome contraction shown in different nuclei. For the Agabus bipustulatus group the RCL data were subjected to statistical analysis using Student’s t-test, but otherwise they are given as approximate values only, to indicate the size relationships of the different pairs of autosomes. Centromere Indices (CI) are not given in detail, but are assigned to their conventional categories. Based on
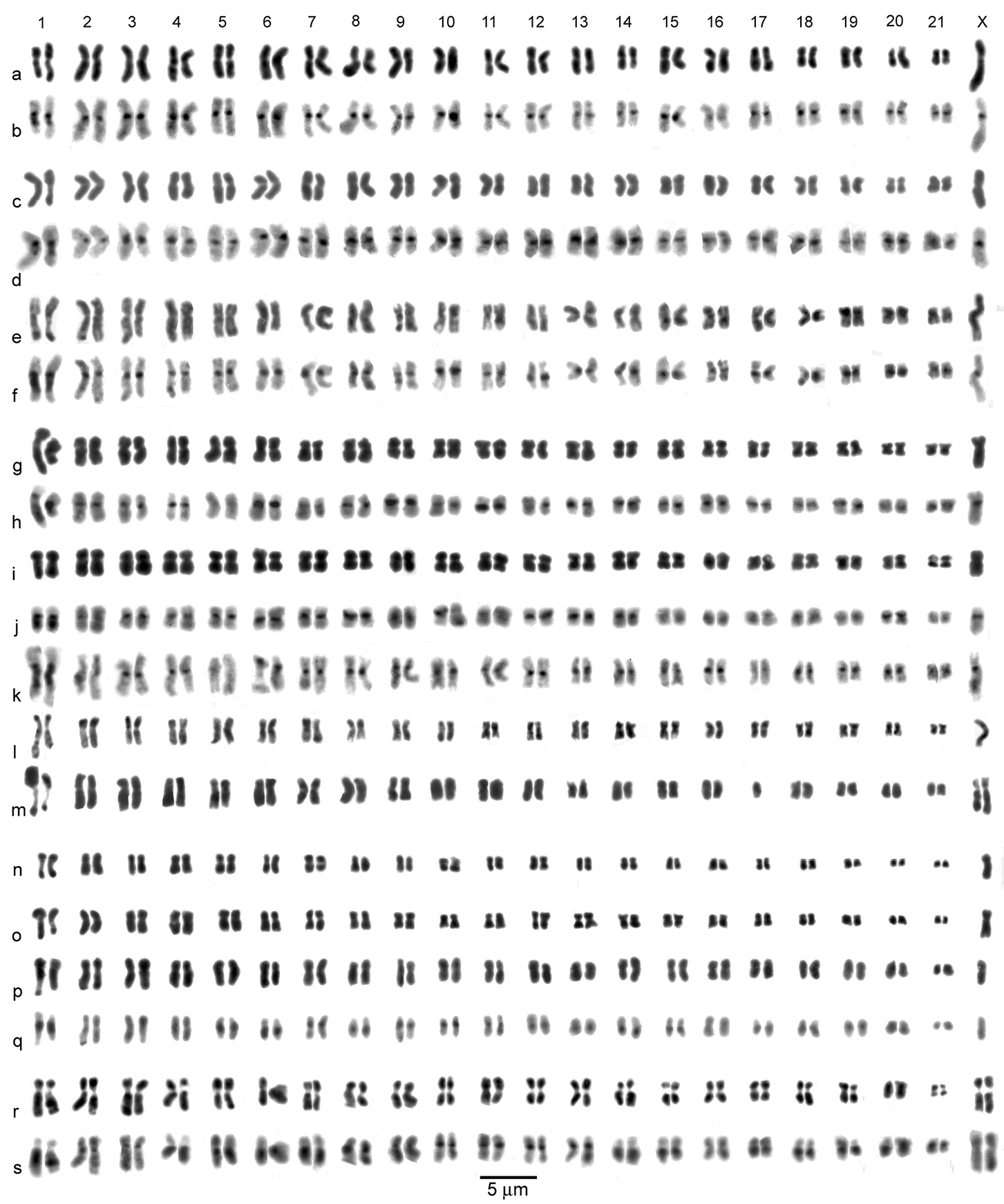
Agabus s. str. (a–e) and Agabus (Acatodes) (f– q), mitotic chromosomes arranged as karyotypes. a Agabus serricornis, ♂, mid-gut, plain b, c Agabus labiatus, ♂, mid-gut b plain c C-banded d, e Agabus labiatus, ♀, mid-gut d plain, e C-banded f, g Agabus congener, ♂, Scotland, testis, C-banded h Agabus congener, ♀, Sweden, mid-gut, C-banded, with 1 B-chromosome i– k Agabus lapponicus, ♂, Sweden, testis i plain j, k C-banded l, m Agabus thomsoni, ♂, mid-gut l plain m the same nucleus C-banded n, o Agabus confinis, ♀, mid-gut, plain n lacking one X chromosome o lacking one replicate each of autosomes 1 and 2 p Agabus sturmii, ♂, mid-gut, plain q Agabus infuscatus, ♂, mid-gut, plain. Bar = 5μm.
Agabus serricornis (Paykull, 1799). Fig. 1a. Published information: none. 2n = 42 + X0 (♂). The RCLs of the autosomes range from about 7.6–2.5, with sharp decreases between pairs 5 (RCL about 6.4) and 6 (RCL about 5), 15 (RCL about 4.5) and 16 (RCL about 3.4), and 20 (RCL about 3.1) and 21 (RCL about 2.5). The X chromosome (RCL about 6.4) is similar in size to pairs 4 and 5. Most of the chromosomes are metacentric to submetacentric, with pairs 8–11 subacrocentric and pairs 15 and 20 more or less acrocentric. Pair 12 has a distinct secondary constriction at the base of its short arm. The X chromosome is subacrocentric, with the centromere clearly nearer the end than in autosomes 4 and 5. No C-banded material is available.
Agabus labiatus (Brahm, 1791). Fig. 1b, c (♂), Fig. 1d, e (♀). Published information: none. 2 n = 42 + X0 (♂), 42 + XX (♀). The autosomes, all either metacentric or submetacentric, have RCLs ranging from about 7.8–2.7, with a fairly gradual decrease along the karyotype, though this is slightly sharper between pairs 5 (RCL about 7.1) and 6 (RCL about 5.9) and 11 (RCL about 4.3) and 12 (RCL about 3.8). The X chromosome is submetacentric and the largest in the nucleus (RCL about 9). Pair 5 have secondary constrictions on the long arm and pair 13 on the short arm. C-banding (Fig. 1c, e) shows a limited development of centromeric C-bands. These are present on autosomes 1, 3–6, 12, 14 and 17–20. The remaining autosomes, and the X chromosome, lack C-bands. Many of the C-bands are very weak, with the strongest bands present on autosomes 5 and 12.
Agabus congener (Thunberg, 1794). Fig. 1f, g (♂), Fig. 1h (♀). Published information: none. 2 n = 42 + X0 (♂), 42 + XX (♀), 1 B-chromosome. The autosomes, all more or less metacentric, have RCLs ranging from about 7–4, with an even size decrease along the karyotype. The submetacentric X chromosome, RCL about 9, is clearly the longest in the nucleus. All the chromosomes have distinct centromeric C-bands, with some variation in strength between pairs, and autosomes 1 and 8 have secondary constrictions which may C-band, especially that on autosome 1. The C-banding reaction of the secondary constriction of autosome 8 is less pronounced, and the constriction may be apparent in only one of the replicates. The Swedish female (Fig. 1h) has one B-chromosome, about as long as autosome 1 and appearing uniformly partly heterochromatic.
Agabus lapponicus (Thomson, 1867). Fig. 1i–k (♂). Published information: none. 2n = 42 + X0 (♂). The karyotype of this species appears indistinguishable from that of Agabus congener.
Agabus thomsoni (J. Sahlberg, 1871). Fig. 1l, m (♂). Published information: none. 2n = 42 + X0 (♂). The karyotype of this species is very similar to those of Agabus congener and Agabus lapponicus, but the longest autosome with a secondary constriction is placed as no. 2 as in this material it appears distinctly shorter than the longest autosome (pair 1). It is possible that additional material would show this not to be the case. As in the preceding two species, the secondary constriction on autosome 8 is more conspicuous in one of the replicates.
Agabus confinis (Gyllenhal, 1808). Fig. 1n, o (♀). Published information: 2n = 40 + “XY” (sex chromosomes not identified) (
Agabus sturmii (Gyllenhal, 1808). Fig. 1p (♂). Published information: 2n = 40 + Xyp (
Agabus infuscatus Aubé, 1838. Figs 1q, 2 (♂). Published information: none. 2n = 42 + neo XY. The autosomes are nearly all either metacentric or submetacentric, but pairs 3 and 17 are subacrocentric. The RCLs of the autosomes range from about 7.9–2.9, and there is a fairly even size decrease along the karyotype, though with slightly sharper decreases between pairs 1 (RCL about 7.9) and 2 (RCL about 6.9), 11 (RCL about 4.3) and 12 (RCL about 3.6), and pairs 18 (RCL about 3.6) and 19 (RCL about 2.9). The subacrocentric X-chromosome (RCL about 7.2) has a distinct gap in its long arm and the Y chromosome, also subacrocentric, is smaller, RCL about 4.6, and matches the X chromosome minus the terminal section of its long arm. This is typical of a neo-XY system where the X chromosome fuses with an autosome to give neo-X, and the same autosome without the X fused to it becomes the neo-Y chromosome. First metaphase of meiosis (Fig. 2) shows 22 bivalents with no suggestion of a B-chromosome behaving differently from the others. Although it is not possible to identify the neo-XY the behaviour of the chromosomes is entirely consistent with a neo-XY system.
a, b Agabus infuscatus testis, first metaphase of meiosis. Bar = 5 μm.
RCL data for this group are given in Table 2.
Agabus bipustulatus group species, Relative Chromosome Length. Mean, 95% confidence intervals, number of chromosomes measured.
| Chromosome | Agabus bipustulatus | Agabus solieri | Agabus nevadensis | Agabus wollastoni | Agabus melanarius |
|---|---|---|---|---|---|
| 1 | 9.11 8.07–10.14 N = 14 |
8.86 6.69–11.03 N = 14 |
8.25 6.41–10.09 N = 10 |
8.56 6.66–10.46 N = 8 |
9.90 8.05–10.95 N = 4 |
| 2 | 9.07 8.15–9.99 N = 14 |
8.11 6.93–9.28 N = 14 |
7.20 6.32–8.08 N = 10 |
7.81 5.61–10.01 N = 8 |
8.75 7.23–10.27 N = 4 |
| 3 | 8.89 8.09–9.70 N = 14 |
8.00 6.37–9.63 N = 14 |
6.85 6.13–7.57 N = 10 |
7.25 4.99–9.51 N = 8 |
8.75 7.23–10.27 N = 4 |
| 4 | 8.29 7.33–9.24 N = 14 |
7.29 6.16–8.42 N = 14 |
6.50 5.87–7.13 N = 10 |
6.63 5.20–8.05 N = 8 |
7.87 7.11–8.64 N = 4 |
| 5 | 7.93 7.20–8.65 N = 14 |
7.00 5.83–8.17 N = 14 |
5.75 4.89–6.61 N = 10 |
6.56 4.88–8.24 N = 8 |
7.50 6.58–8.42 N = 4 |
| 6 | 7.54 6.82–8.26 N = 14 |
6.71 5.54–7.88 N = 14 |
5.40 4.59–6.21 N = 10 |
6.19 4.92–7.45 N = 8 |
6.50 5.58–7.42 N = 4 |
| 7 | 6.79 5.86–7.71 N = 14 |
6.07 4.73–7.42 N = 14 |
5.15 4.29–6.01 N = 10 |
6.00 4.14–7.86 N = 8 |
7.25 6.22–8.28 N = 4 |
| 8 | 6.54 5.63–7.44 N = 14 |
5.96 4.75–7.18 N = 14 |
4.90 4.19–5.61 N = 10 |
5.50 4.03–6.97 N = 8 |
6.50 5.58–7.42 N = 4 |
| 9 | 6.25 5.66–6.84 N = 14 |
5.68 4.64–6.71 N = 14 |
4.70 3.94–5.46 N = 10 |
5.44 3.87–7.01 N = 8 |
6.88 6.48–7.27 N = 4 |
| 10 | 6.25 5.68–6.82 N = 14 |
5.39 4.40–6.38 N = 14 |
4.35 3.60–5.11 N = 10 |
4.94 3.50–6.38 N = 8 |
6.63 5.86–7.39 N = 4 |
| 11 | 5.71 5.21–6.22 N = 14 |
5.25 4.25–6.25 N = 14 |
4.20 3.66–4.74 N = 10 |
4.63 3.35–5.90 N = 8 |
6.63 5.86–7.39 N = 4 |
| 12 | 5.75 5.23–6.27 N = 14 |
5.29 4.39–6.18 N = 14 |
3.85 3.26–4.44 N = 10 |
4.69 3.51–5.87 N = 8 |
6.38 5.18–7.57 N = 4 |
| 13 | 5.71 5.28–6.15 N = 14 |
4.93 4.05–5.81 N = 14 |
3.95 3.64–4.26 N = 10 |
4.31 3.11–5.51 N = 8 |
6.00 4.16–7.84 |
| 14 | 5.86 5.31–6.41 N = 14 |
4.89 4.07–5.71 N = 14 |
3.75 3.36–4.14 |
3.94 2.79–5.09 N = 8 |
6.38 5.37–7.38 N = 4 |
| 15 | 5.39 4.91–5.88 N = 14 |
5.07 4.18–5.96 N = 14 |
3.45 2.96–3.94 N = 10 |
3.75 2.83–4.67 N = 8 |
6.00 5.74–6.26 N = 4 |
| 16 | 5.04 4.34–5.73 N = 14 |
4.54 3.66–5.41 N = 14 |
3.55 3.09–4.01 N = 10 |
3.44 2.59–4.29 N = 8 |
5.63 4.86–6.39 N = 4 |
| 17 | 4.79 4.22–5.35 N = 14 |
4.29 3.46–5.11 N = 14 |
3.33 3.00–3.67 N = 9 |
3.25 2.28–4.22 N = 8 |
5.00 4.74–5.26 N = 4 |
| 18 | 4.25 3.77–4.73 N = 14 |
4.04 3.21–4.86 N = 14 |
3.05 2.62–3.48 N = 10 |
3.13 2.11–4.14 N = 8 |
5.50 4.58–6.42 N = 4 |
| 19 | 4.21 3.84–4.58 N = 14 |
3.93 3.26–4.60 N = 14 |
2.70 2.19–3.21 N = 10 |
2.63 1.89–3.36 N = 8 |
4.88 4.48–5.27 |
| 20 | 3.75 3.36–4.14 N = 14 |
3.36 2.71–4.00 N = 14 |
2.50 2.16–2.84 N = 10 |
2.38 1.41–2.54 N = 8 |
4.13 3.72–4.52 N = 4 |
| 21 | 3.25 2.77–3.73 N = 14 |
2.54 2.04–3.04 N = 14 |
2.05 1.62–2.48 N = 10 |
1.87 1.21–2.54 N = 8 |
2.75 1.95–3.55 N = 4 |
| X | 11.43 8.95–13.91 N = 7 |
8.86 5.69–12.02 N = 7 |
7.83 6.40–9.27 N = 5 |
8.25 5.75–10.75 N = 6 |
8.33 3.16–13.50 N = 3 |
Agabus bipustulatus (Linnaeus, 1767). Fig. 3a–f. Published information: 2n = 40 + Xyp (
Agabus bipustulatus var. solieri Aubé, 1837. Fig. 3g–k. Published information: none. 2n = 42 + X0 (♂), 42 + XX (♀). All the preparations illustrated are from the Swiss Alps, and are chosen because good plain and C-banded preparations were obtained from the same nuclei. The nuclei shown in Fig. 3g–j are more condensed than the typical Agabus bipustulatus shown, but the one in Fig. 3k shows a comparable degree of condensation. These karyotypes show no obvious difference from those of typical Agabus bipustulatus. The dark area at the end of the X chromosome in Fig. 3k is where it overlapped one of the autosomes in the preparation. The extreme size difference between the two replicates of autosome 1 in Fig. 3g, h is very striking, but C-banding (Fig. 3h) shows that this size difference is entirely due to the degree of expansion of the secondary constriction.
Agabus nevadensis Håkan Lindberg, 1939. Fig. 3l, m. Published information: none. 2n = 42 + X0 (♂), 42 + XX (♀) The preparations are from old material in R. B. Angus’ archive, and no C-banding is available. The heavy short arm of one replicate of autosome 1 in Fig. 3m is the result of its lying on top of dark material. The sizes and shapes of these chromosomes show no detectable differences from those of Agabus bipustulatus and Agabus bipustulatus var. solieri.
Agabus wollastoni Sharp, 1882. Fig. 1n, o. Published information: none. 2n = 42 + X0 (♂). As with Agabus nevadensis, this is archive material and no C-banding is available. Only two karyotypes could be obtained, both from rather condensed nuclei, but the general arrangement of the chromosomes is very similar to, if not identical with, those of the species already discussed.
Agabus melanarius Aubé, 1837. Fig. 3p–s. Published information: none. 2n = 42 + X0 (♂), 42 + XX (♀). The general layout of the karyotype is very similar to those of the Agabus bipustulatus complex described above, but there appear to be more secondary constrictions. Thus in the female (Fig. 3s), where the C-banding is better displayed, secondary C-bands are clear in autosomes 1, 3, 6, 7 and 14, and even in the male (Fig. 3q) the secondary C-bands are clear in autosomes 1, 6 and 14.
Agabus (Gaurodytes) part 1, the Agabus bipustulatus group, mitotic chromosomes arranged as karyotypes. a– f Agabus bipustulatus: a–d ♂, Inari, testis a plain b the same nucleus C-banded c plain d the same nucleus C-banded e, f ♂, Woolmer, mid-gut e plain f the same nucleus C-banded g– k Agabus bipustulatus var. solieri: g, h ♂, Moiry, testis g plain h the same nucleus C-banded i–j, k Illsee, ♂, testis i plain j the same nucleus C-banded k a different nucleus C-banded l, m Agabus nevadensis, Sierra Nevada, mid-gut, plain, l ♂, m♀ n, o Agabus wollastoni, ♂, Madeira, testis, plain; p–s Agabus melanarius: p, q ♂, testis p plain q the same nucleus C-banded r, s ♀, ovary, r plain, s the same nucleus C-banded. Bar = 5 μm.
Agabus biguttatus (Olivier, 1795). Fig. 4a, b. Published information: 2n = 42 + X0 (♂), 22 + XX (♀) (
Agabus binotatus Aubé, 1837. Fig. 4c. Published information: none. 2n = 42 + X0 (♂). The karyotype of this species appears very similar to that of Agabus biguttatus, with a similar spread of RCLs. However, autosomes 14–21 are clearly less metacentric than in Agabus biguttatus, in some cases approaching subacrocentric. The X chromosome, RCL about 8.5, is clearly the largest in the nucleus, thus distinctly larger than in Agabus biguttatus.
Agabus affinis (Paykull, 1798). Fig. 4d. Published information: none. 2n = 42 + X0 (♂). The RCLs of the autosomes range from about 8–2.7, with an abrupt size decrease between pair 4 (RCL about 7.4) and pair 5 (RCL about 5.4), but otherwise with a gradual decrease. Most to the autosomes are either metacentric or submetacentric, but autosomes 12, 17, 20 and 21 are subacrocentric. The X chromosome is submetacentric, RCL about 6. No C-banded material is available.
Agabus unguicularis (Thomson, 1867). Fig. 4e. Published information: none. 2n = 42 + X0 (♂). The RCLs of the autosomes range from about 10–2.4. There is an abrupt size decrease between pairs 2 and 3 (RCLs about 9.4 and 7.6) and pairs 3 and 4 (RCL of pair 4 about 6.5), but apart from that the size decrease is fairly even. Most of the autosomes are metacentric or almost so, but a few are clearly submetacentric. The X chromosome, RCL about 6.5, is similar in size to autosome pair 4, but much more clearly submetacentric. No C-banded material is available.
Agabus ramblae Millan et Ribera, 2001. Fig. 4f. Published information: none. 2n = 42 + X0 (♂), 42 + XX (♀).The RCLs of the autosomes range from about 7–2.9, with a fairly even decrease in length along the karyotype. The autosomes are a mixture of metacentrics and submetacentrics (some at the extreme end of the range), with autosomes 10–12, 15, 16 and 20 subacrocentric. The X chromosome is about the same size as autosome 1, but more clearly submetacentric. No C-banded material is available.
Agabus conspersus (Marsham, 1802). Fig. 4g. Published information: 2n = 38 + XY (
Agabus nebulosus (Forster, 1771). Fig. 4h, i. Published information: none. 2n = 42 + X0 (♂), 42 + XX (♀). The general layout of the karyotype in terms of RCLs of the autosomes is very similar to that of Agabus conspersus. Autosome 3 has a similar secondary constriction in its long arm, but the small chromosome with the terminal apparent NOR is relatively larger than in Agabus conspersus, and is placed as pair 12 as against 15. The X chromosome, RCL about 7.3, appears relatively larger than that of Agabus conspersus, and is metacentric. The Tenerife specimen whose chromosomes are shown in Fig. 4i is of a form whose dark pronotal spots are absent or scarcely apparent, but the chromosomes clearly associate it with the British well-spotted Agabus nebulosus rather than Agabus conspersus which lacks the pronotal spots.
Agabus adpressus Aubé, 1837. Fig. 4j–l. Published information: none. 2n = 42 + XY (♂). The autosomes are all either metacentric or submetacentric, with RCLs ranging from about 7.2–3.1 and with an even decrease in size along the karyotype. Autosome 2 has a secondary constriction in its long arm and autosome 8 has one in its short arm. The X chromosome is submetacentric (almost metacentric), about as long as autosome 1. The Y chromosome, RCL about 5, looks like the X chromosome with most of one arm missing. C-banding (Fig. 4l) shows considerable variation in the centromeric C-bands of the autosomes. Autosome 1 lacks any C-band, 2 and 3 have strong C-bands and 4 has a weak one. Autosome 5 lacks a C-band and that on autosome 6 is very weak. Autosomes 7–9 have strong centromeric C-bands and 10–13 have weaker ones. Pair 14 has very weak bands. Pairs 15–21 have strong C-bands. The secondary constriction of autosome 2 shows as a C-band, but that of autosome 8 appears to be merged with the strong centromeric C-band. The sex chromosomes both have very large strong centromeric C-bands, which is a powerful piece of evidence that this is a neo-XY system rather than an X0 system and a B-chromosome. Unfortunately no meiotic preparation is available.
Agabus (Gaurodytes) part 2, mitotic chromosomes arranged as karyotypes. a, b Agabus biguttatus, ♂, mid-gut, plain: a El Noqra b Giara di Gesturi; c Agabus binotatus, ♂, mid-gut, plain d Agabus affinis, ♂, mid-gut, plain e Agabus unguicularis, ♂, mid-gut, plain f Agabus ramblae, ♂, Murcia, testis, plain g Agabus conspersus, ♂, mid-gut, plain h, i Agabus nebulosus, ♂, mid-gut, plain h Cuckmere i Tenerife j–l Agabus adpressus, ♂, mid-gut j plain k, l the same nucleus k plain l C-banded. Bar = 5 μm.
Colymbetes fuscus (Linnaeus, 1758). Fig. 5a, b. Published information: 2n = 35–37 (♀) (
Colymbetes paykulli Erichson, 1837. Fig. 5c, d. Published information: 18 pairs including Xyp? (
Colymbetes piceus Klug, 1834. Fig. 5e. Published information: 2n = 40 + X0 (♂), 40 + XX (♀) (
Colymbetes striatus (Linnaeus, 1758). Fig. 5f, g. Published information: 19 - 21 pairs + Xyp? (
Colymbetes (a–g) and Rhantus (h–t), mitotic chromosomes arranged as karyotypes. a, b Colymbetes fuscus, ♂, mid-gut a Pinail, plain b Wisley, C-banded c, d Colymbetes paykulli, ♂, midgut, Mullsjö c plain d C-banded e Colymbetes piceus, ♂, Egypt, mid-gut, plain f, g Colymbetes striatus, ♂, mid-gut, plain h, i Rhantus grapii, ♂, mid-gut, plain j, k Rhantus exsoletus, ♂, mid-gut, plain j Studland Heath k Gayton Thorpe Common l, m Rhantus frontalis, ♂, plain l mid-gut, Gayton Thorpe Common m testis, Thompson Common n–p Rhantus suturalis, ♂ n, o mid-gut, Staines Moor n plain o C-banded p testis, Ras Az Zawr, Kuwait, plain q–t Rhantus suturellus, plain q♂, testis, Pinail r–t Studland Heath r♀ mid-gut s♂ mid-gut with 1 Bchromosome t♀ mid-gut with 1 B-chromosome. Bar = 5 μm.
Rhantus grapii (Gyllenhal, 1808). Fig. 5h, i. Published information: none. 2n = 44 + X0 (♂), 44 + XX (♀). The RCLs of the autosomes range from about 9.6–2.5, with an even decrease in chromosome length along the karyotype, apart from sharp decreases in size between pairs 1 and 2 (RCL about 7) and between pair 21 (RCL about 5.5) and pair 22 (RCL about 1.2). Most of the autosomes are more or less metacentric, but pairs 4, 5, 7, 10–12, 14, 16–18 and 20 are clearly submetacentric and pairs 21 and 22 are subacrocentric. Autosome 9 has a secondary constriction towards the end of its long arm. The X chromosome, RCL about 7.7, is the second to longest in the nucleus and is submetacentric. No C-banded material is available.
Rhantus exsoletus (Forster, 1771). Fig. 5j, k. Published information: 20 pairs + Xyp (
Rhantus frontalis (Marsham, 1802). Fig. 5l, m. Published information (as Rhantus notatus F.): 22 pairs including Xyp (
Rhantus suturalis (Macleay, 1825). Fig. 5n–p. Published information: none. 2n = 44 + X0 (♂). The RCLs of the autosomes range from about 6.1–3.5. The rate of decrease along the karyotype is very even with many of the adjacent pairs appearing more or less the same size. Most of the autosomes are more or less metacentric but pairs 13, 14 and 22 are clearly submetacentric and pair 20 is subacrocentric. C-banding (Fig. 5o) shows all the chromosomes with centromeric C-bands, of varying strengths. Pairs 1, 8 and 14 have secondary constrictions on their short arms. The X chromosome, RCL about 5.8, is metacentric with a rather weak centromeric C-band. The Kuwaiti material (Fig. 5p) shows no differences from the British.
Rhantus suturellus (Harris, 1828). Fig. 5q–t. Published information: none. 2n = 40 + X0 (♂), 40 + XX (♀), sometimes with 1 B-chromosome. The RCLs of the autosomes range from about 7–2.7, with a fairly even decrease in chromosome length along the karyotype. Autosomes 5, 7, 9, 11, 12, 15 -17, 19 and 20 are clearly submetacentric, with the remainder more or less metacentric. Pairs 4, 8 and 9 have secondary constrictions in their short arms. The X chromosome, RCL about 5.3, is metacentric and similar to chromosomes 4–8. No C-banded material is available. This karyotype is unusual in having a B-chromosome, a small metacentric, RCL about 3, which has so far been found in Studland Heath material. The first Studland Heath material, in 1993, comprised a male with a B-chromosome and a female without one, giving the impression that this species had an XY sex chromosome system. However, the 2000 material, a mail from Pinail lacking the B-chromosome and a female from Studland Heath with the B-chromosome, revealed the true nature of the situation.
In considering the data presented here, two aspects are of particular note: the extent to which the different genera have characteristic karyotypes and details of any deviations from generic karyotypes; and the extent to which the karyotypes of related species show clear differences.
In Agabus 18 of the 20 species reported have a karyotype involving 21 pairs of autosomes and sex chromosomes which are X0 (♂) and XX (♀), but the remaining 2, Agabus infuscatus and Agabus adpressus, have 21 pairs of autosomes and sex chromosomes which are XY (♂) and XX (♀). These two species are not closely related (they are placed in different subgenera), but appear to have evolved similar neo-XY sex chromosomes. What makes this particularly surprising is that, since the development of a neo-XY system involves fusion of the original X chromosome with an autosome, there should be an initial reduction by one in the number of pairs of autosomes. However, both the species involved here show no such reduction, so have presumably undergone fission of one autosome to give two and hence restore the original number. It may be noted that
Among the Agabus species reported here, there are two groups of particularly close relatives, Agabus congener, lapponicus and thomsoni, and the Agabus bipustulatus group. Agabus congener and lapponicus show no interspecific chromosomal differences despite a good number of high-quality preparations. Agabus thomsoni may show a slight difference in the RCL of the longest secondary constriction-bearing autosome, but more material would be needed to confirm this.
The Agabus bipustulatus group comprises Agabus melanarius and the Agabus bipustulatus complex within which the overriding impression from the present investigation is the extreme similarity between the karyotypes of the species. In the case of Agabus bipustulatus and Agabus bipustulatus var. solieri this is not surprising as these are regarded as conspecific. The case of Agabus nevadensis is perhaps more interesting as this is currently regarded as a distinct species in spite of the lack of clear morphological characters to distinguish it from Agabus bipustulatus. The karyotype of Agabus wollastoni also shows no obvious difference from those of the other species, but in this case the species does have a very clear morphological character to distinguish it from Agabus bipustulatus–the inner anterior tarsal claw of the male is simple, not expanded to give the “scooped-out” appearance characteristic of Agabus bipustulatus, solieri and nevadensis. Only Agabus melanarius, not really a member of the Agabus bipustulatus complex, shows some karyotype differences, most clearly in the more extensive development of heterochromatic (C-banding) regions on the chromosomes. These findings may be considered in the light of the phylogenetic trees obtained by
Examination of the material of Agabus bipustulatus, solieri and nevadensis included in their study shows how they came to their conclusions as to their taxonomic status. They are concerned with forms in which the primary reticulation (the fine meshes inside the larger elongate secondary meshes) is progressively reduced. These forms are referred to the varieties dolomitanus Scholz, 1935, falcozi Guignot, 1932, kiesenwetteri Seidlitz 1887 and pyrenaeus Fresneda and Hernando, 1989. The most striking thing is that these various solieri forms come out in a number of different places, often with ordinary bipustulatus from neighbouring areas. Agabus nevadensis, with its very restricted distribution, almost inevitably comes out in only one place, but very closely associated with a population of solieri (kiesenwetteri) from France. The claim of Agabus nevadensis to species status appears weak. The mitochondrial DNA separation is very slight, the karyotype appears identical with those of other Agabus bipustulatus forms, and the morphological characteristics are less clear than those of solieri.
The case of Agabus wollastoni is interesting. This species is isolated on Madeira and has had time to diverge from other Agabus bipustulatus, both in its mitochondrial DNA and also in its morphology–simple inner anterior tarsal claws of males, and generally larger size. Only the chromosomes show no difference.
The four species of Colymbetes share the same basic karyotype with 2n = 40 + X0 (♂), with the X chromosome a large more or less metacentric. There are minor differences in the RCL sequences between the species, which may or may not stand up to more detailed analysis if more material becomes available. Autosome 1 of Colymbetes striatus appears larger than in the other species.
The karyotypes of the Rhantus species are interesting in showing two different numbers, with 2n = 40 + X0 (♂) in Rhantus exsoletus and Rhantus suturellus, but 2n = 44 + X0 (♂) in the other species studied. Interestingly, this number difference does not reflect the subgeneric classification. The B-chromosome of Rhantus suturellus is interesting in that it could be confused with a neo-XY sex chromosome system comparable with that of Agabus infuscatus and Agabus adpressus.
The Kuwaiti material of Rhantus suturalis is interesting as it shows no differences from British material.
We thank Anders Nilsson, Marcus Drotz, Garth Foster, Ignacio Ribera and Andres Millan for collecting and sending some of the material used in this study.