(C) 2013 Ekaterina Gornung. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The karyotype of a sphaerodactylid gecko Euleptes europaea (Gené, 1839) was assembled for the first time in this species. It is made of 2n = 42 gradually decreasing in size chromosomes, the highest chromosome number so far acknowledged in the family Sphaerodactylidae. The second chromosome pair of the karyotype appears slightly heteromorphic in the male individual. Accordingly, FISH with a telomeric probe revealed an uneven distribution of telomeric repeats on the two homologues of this pair, which may be indicative of an XY sex-determination system in the species, to be further investigated.
Sauria, Gekkota, karyotype, chromosomal evolution, telomeric repeats, XY male heterogamety
The Italian Gekkotan fauna includes four species: two gekkonid species – Mediodactylus kotschyi (Steindachner, 1870) and Hemidactylus turcicus (Linnaeus, 1758), a phyllodactylid gecko Tarentola mauritanica (Linnaeus, 1758), and a sphaerodactylid Euleptes europaea (Gené, 1839) (
In Europe, Euleptes Fitzinger, 1843 is described from at least the early Miocene; the single modern species, Euleptes europaea, is a relic endemic of the western Mediterranean region which survived during isolation on the Corso-Sardinian microplate (
Euleptes europaea remains the only gecko of the Italian fauna, which has not been characterized cytogenetically. It is not surprising, since of approximately 1, 000 species of Geckonids, in the broad sense, karyotypes have been described for less than 10% of them (
We analyzed a limited sample - one male, one female, and one juvenile - from a population of the island of Santa Maria near the north coast of Sardinia. The animals were handled according to the European Code of Practice for the housing and care of animals used in scientific procedures (Council of Europe 1986). Analyzed specimens (voucher numbers: EUL1 male, EUL2 juvenile, EUL3 female) are preserved in 70% ethanol and are housed in the herpetological collection of the Dipartimento di Biologia e Biotecnologie “Charles Darwin” Università di Roma “La Sapienza” (CEAC).
Metaphase plates were prepared from bone marrow, intestinal, and testicular cells using standard air-drying method after injection of 1:1000 solution Vinblastine Sulphate, Velbe® (Lilly) as antimitotic solution. The slides were colored with 5% Giemsa solution. For each individual, about 20 metaphase plates were studied and photographed. The telomeric probe was commercially synthesized as two oligonucleotides (GGGTTA)7 and (TAACCC)7 both end-labeled with Cy3 (Bio-Fab Research). The oligonucleotides were dissolved (2 ng/µL) in a hybridization mix made up of 5% Dextran sulphate, 2XSSC, and 5 µg/µL sonicated salmon DNA. For FISH, standard procedures for the hybridization of repetitive sequences (
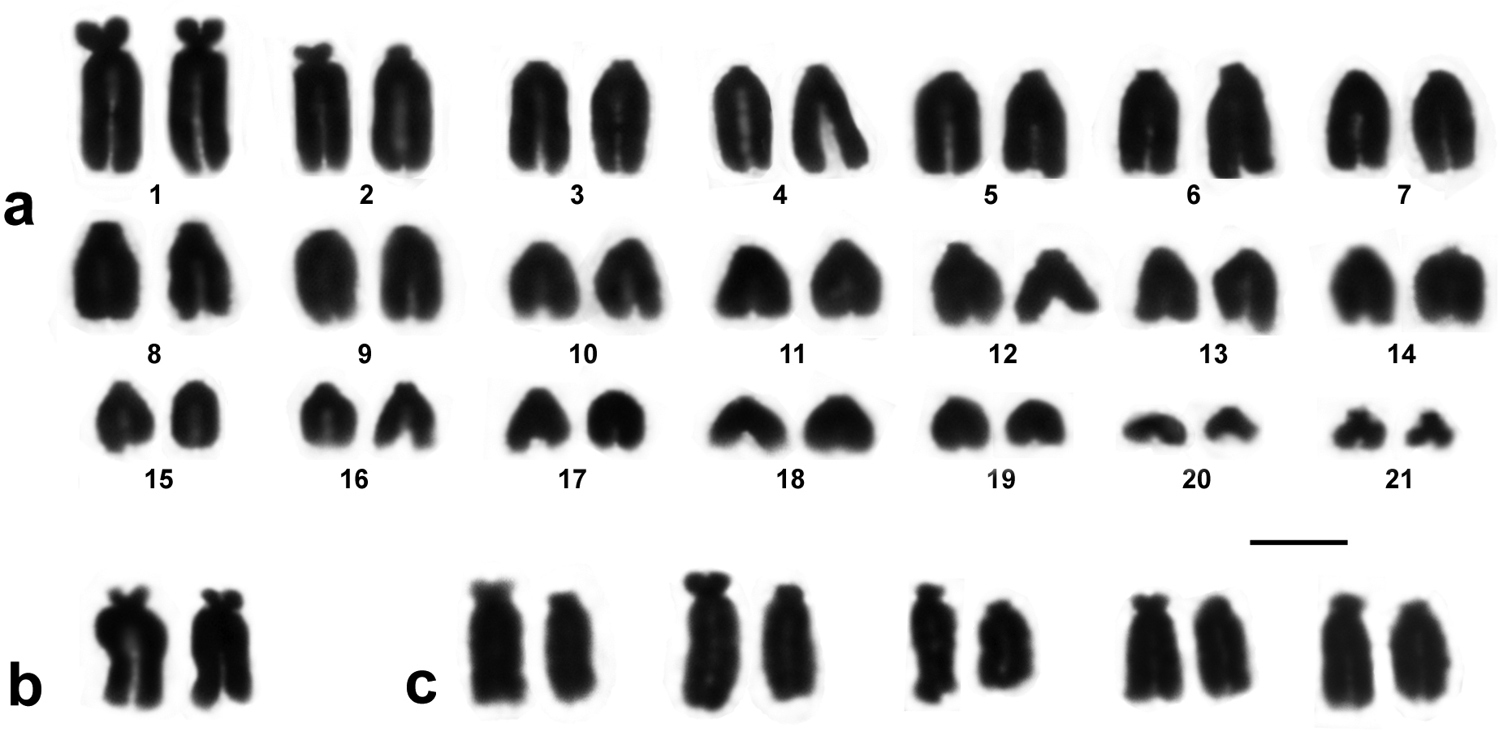
The karyotype of Euleptes europaea is composed of 21 chromosome pairs gradually decreasing in size (Fig. 1a). There is no pronounced subdivision of the chromosome complement into macro- and microchromosomes; 17 chromosome pairs may be considered telocentric chromosomes: tiny short arms, visible in some of more elongated chromosomes, are not taken into account for the fundamental number. The minute chromosomes № 20 and № 21 are telocentric, while the smallest pair of the karyotype is definitely biarmed. The largest chromosomes of the complement (pairs № 1 and № 2) are also biarmed, precisely, subtelocentric. However, both homologues of the chromosomes № 2 had short, similar in size true arms only in the female individual (Fig. 1b). In the male, one of the homologues of chromosomes № 2 showed somewhat greater overall compactness and smaller or more contracted short arms in most metaphases after conventional Giemsa staining (Fig. 1c). The degree of this heteromorphism was relevant enough to be worth noting: the average centromeric index of the two homologues of this pair was 14.7% and 8.3%.
Chromosome complement of Euleptes europaea from Sardinia. a 2n = 42 male karyotype b homomorphic chromosomes 2 (female); c – examples of heteromorphic chromosomes №2 (male). Bar = 5 µm.
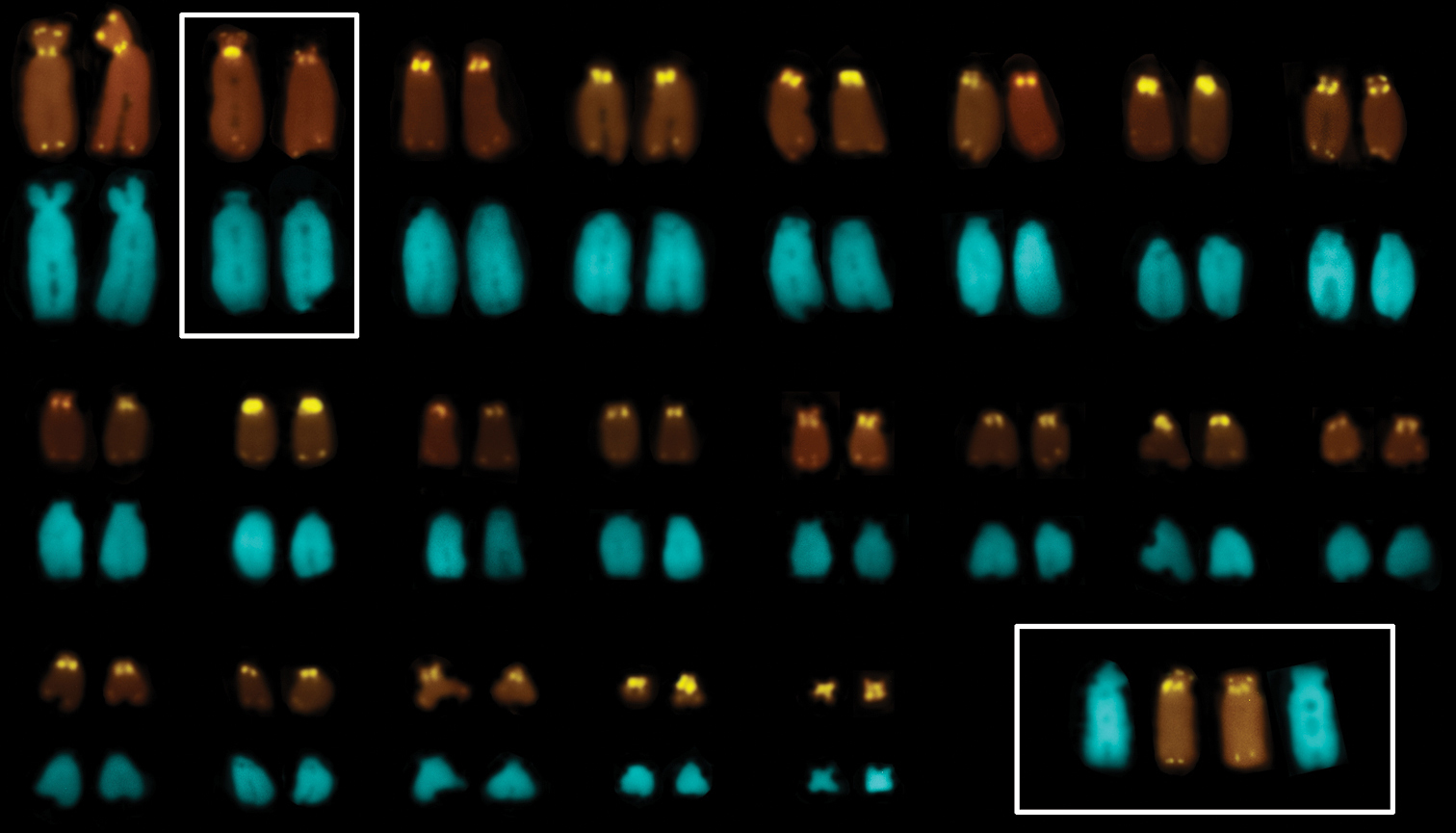
FISH with a telomeric probe detected all ordinary telomeric sites of the chromosomes. The present results are in accordance with previously obtained data in Gonatodes taniae Roze, 1963, the only other sphaerodactylid species, in which chromosomal distribution of telomeric sequences has been studied so far (
A karyotype of Euleptes europaea after FISH with a telomeric probe (upper array) and DAPI-staining (lower array); slightly heteromorphic chromosomes № 2 are framed; the same chromosome pair of a female is shown in the insert below.
The ITS sites at centromeres have been described in many different taxonomic groups (
In summary, the karyotype of Euleptes europaea looks quite unusual if compared with other records available in the family Sphaerodactylidae, and the chromosome number is the highest among all species of the family presently studied. Since the phylogenetic position of Euleptes within Sphaerodactylidae is uncertain, we provide a comparative analysis of all-encompassing data. The genus Euleptes falls in a poorly supported assemblage of genera without clear relationships with each other, which includes the following species-poor Afro-Asian genera: Pristurus Rüppell, 1835, endemic to Middle East and Arabia, the Asiatic Teratoscincus Strauch, 1863, and the Moroccan Quedenfeldtia Boettger, 1883, as well as the neotropical species-rich Aristelliger Cope, 1861 (
The family Sphaerodactylidae includes also one well supported major clade, which comprises five genera of the neotropical sphaerodactylid lizards (Coleodactylus Parker, 1926; Gonatodes Fitzinger, 1843; Lepidoblepharis Peracca, 1897; Pseudogonatodes Ruthven, 1915, and Sphaerodactylus Wagler, 1830) (see
Another outcome of the present study is a possible male chromosome heteromorphism in Euleptes europaea. However, provided the extremely limited sample presently examined, chromosome polymorphism unrelated to sex is possible, as well. If the present data in Euleptes europaea actually reflect the XX/XY sex determination system, which is still to be corroborated, it would be indicative of rather new or undifferentiated sex chromosomes. The available cytogenetic data on sex chromosomes in Sauria are rare, but give an idea of how different may be the morphology and composition of sex chromosomes in different species with male (XX/XY) or female (ZZ/ZW) heterogamety (
The main conclusions of the present analysis are: 1) the diploid chromosome number in Sphaerodactylidae may reach 2n = 42, the uppermost value so far observed in the family, as well as one of the highest diploid numbers among all Gekkotan lizards (acknowledged maximum is 2n = 46 in Thailand house gecko, Cosymbotus platyurus (Schneider, 1792) (classified also as Hemidactylus platyurus (Schneider, 1792)) according to
This work was supported by funds “Progetti di Ricerca, Università di Roma “La Sapienza” (grants to R.C.). The text was checked by a professional native English editor.