(C) 2013 Artem P. Lisachov. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
DAPI staining of the metaphase chromosomes pretreated with barium hydroxide generates a C-like banding pattern. In this work a protocol for visualizing similar pattern at the synaptonemal complex (SC) spreads after immunostaining is suggested. This method was used to visualize centromeric and sex heterochromatin at the SC spreads of guppy fish (Poecilia reticulata Peters, 1859). The efficiency of this method was further confirmed at SC spreads of the northern red-backed vole (Myodes rutilus (Pallas, 1779)), the guinea pig (Cavia porcellus (Linnaeus, 1758)), and the pigmy shrew (Sorex minutus Linnaeus, 1766).
Synaptonemal complexes, pachytene, DAPI, C-banding
Immunofluorescent analysis of synaptonemal complexes (SC) is widely performed in medical and comparative cytogenetics to study chromosome synapsis and recombination in humans with chromosomal abnormalities (
Centromeres are used as an important marker in SC karyotyping. For instance, centromeres are commonly detected by human autoantibodies to human centromere proteins (CREST serum from human scleroderma patients). These proteins are conservative and human antibodies may be used to study various species (
In this study a simple protocol was designed to obtain C-like banding on SC spreads and visualize centromeres without the use of antibodies. This method is cheaper and applicable if antibodies fail to detect centromeres because of a large phylogenetic distance. This method is a modified DAPI-staining technique combined with pretreatment commonly used for C-banding. The proposed method was tested on guppy fish. The efficiency of this method was confirmed in three of five examined mammal species. Previously, the DAPI staining after barium hydroxide treatment was shown to visualize C-like banding on the metaphase chromosomes of fish (Russo, Rocco et al. 1999), plants (
Mammal spermatocytes were prepared according to the prescribed method of (
This method also yielded satisfactory results for guppy spermatocytes. However, more accurate results were obtained using the method of (
The slides with guppy spermatocytes were permeabilized by the incubating in 10 mM sodium citrate solution with 0.1% Tween-20 at 95° C for 20 min before immunostaining was performed. The slides were then cooled to room temperature for 20 min and rinsed in PBS with 0.1% Tween-20 for 2 min twice. Immunostaining was performed according to the protocol described in (
The preparations were photographed using Axioplan 2 Imaging microscope with a CCD camera (CV M300, JAI Corporation, Japan), CHROMA filter sets, and an ISIS4 image processing package (MetaSystems GmbH, Germany). The coverslips were carefully removed after the photographs were taken. The preparations were washed in 2×SSC for 5 min to remove the antifade solution and then dehydrated in ethanol series 70%, 80% and 100% for 3 minutes in each. The preparations were then air-dried and stored in 0, 2 N HCl at room temperature for 20 min to 30 min. The slides were transferred to saturated barium hydroxide solution at 55°C for 1 min to 10 min. Afterward, the preparations were incubated in 2×SSC at 55°C to 60°C for 60 min. The preparations were re-mounted in the antifade solution with DAPI and the cells were re-photographed.
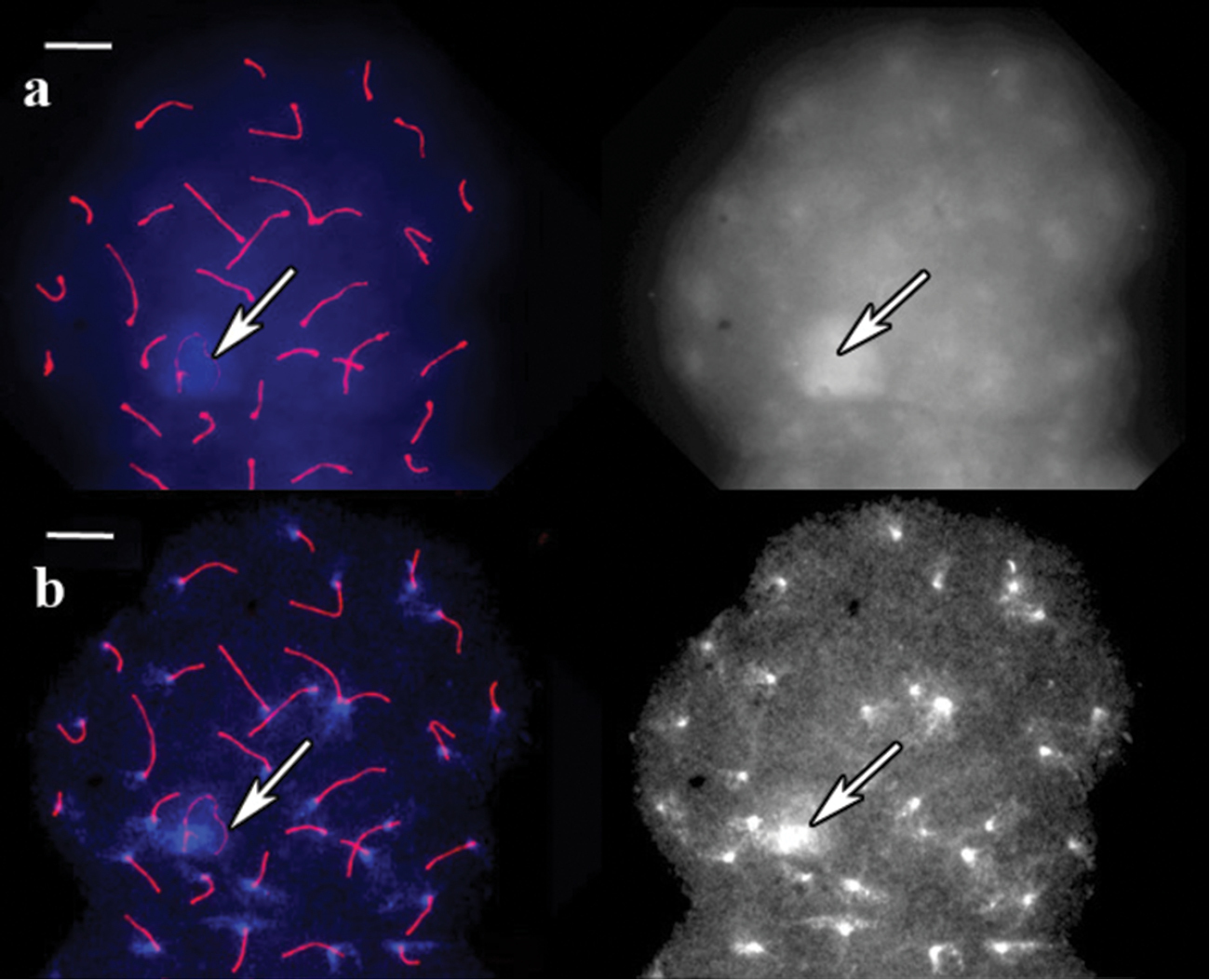
In vole, guinea pig and pigmy shrew, the centromeres were marked by antibodies and DAPI signals. In vole, DAPI-stained pachytene cells untreated with barium hydroxide produced a unique banding pattern for each chromosome. This banding pattern is visible even without image contrast adjustment, and bright bands correspond to centromere antibody signals (Fig. 1a). However, the centromeric bands of most chromosomes were indistinguishable by their brightness from the interstitial ones. After the incubation in barium hydroxide for 5 minutes, all the bands except the centromeric bands and the sex heterochromatin faded and disappeared (Fig. 1b).
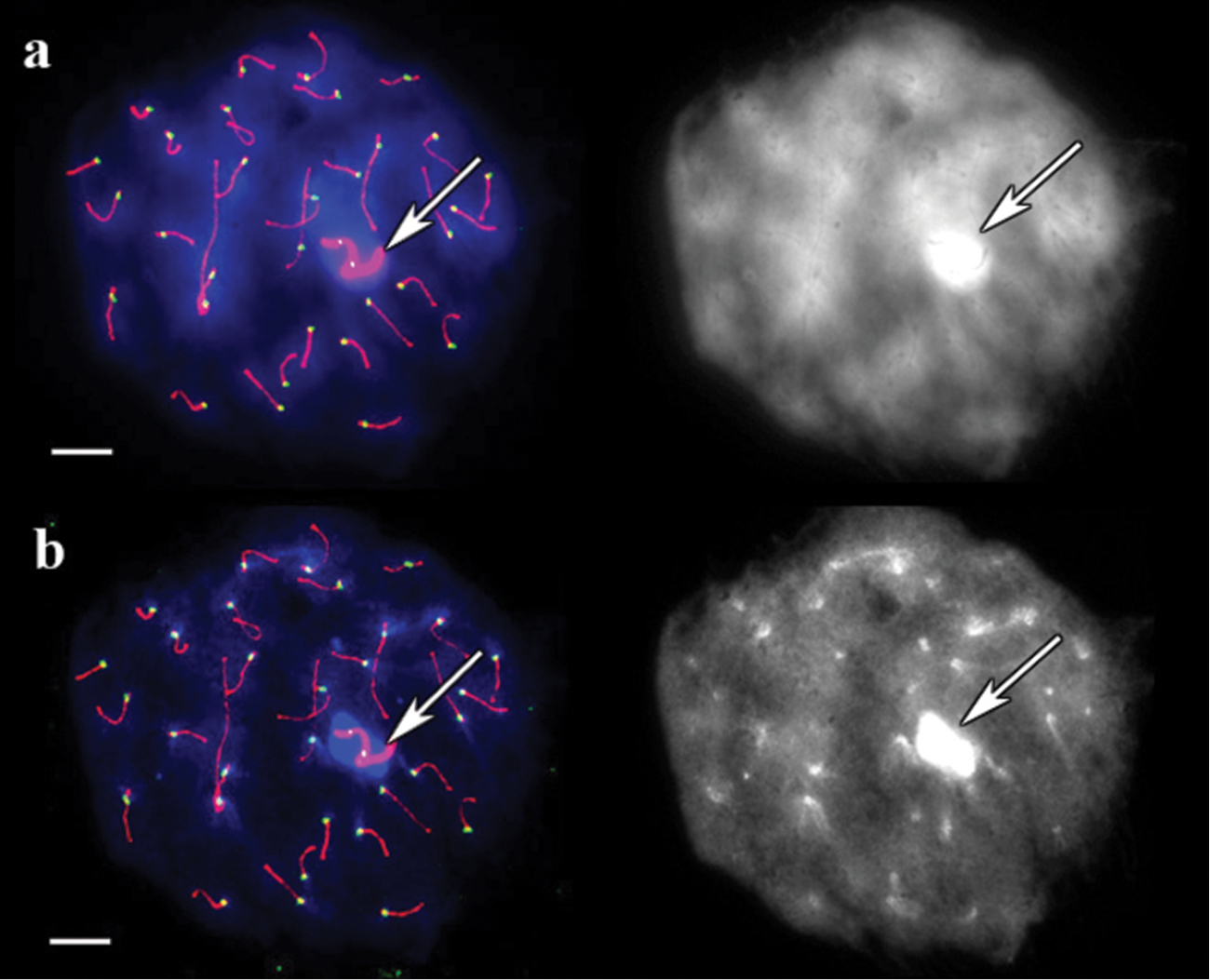
In guinea pig, DAPI-stainied pachytene chromosomes untreated with barium hydroxide produced a weak differential pattern that only became visible after image contrast was adjusted, and the centromeres were indistinguishable (Fig. 2a). After barium hydroxide treatment for 5 minutes, a distinct DAPI signal at the centromeres and over the sex bivalent was observed (Fig. 2b).
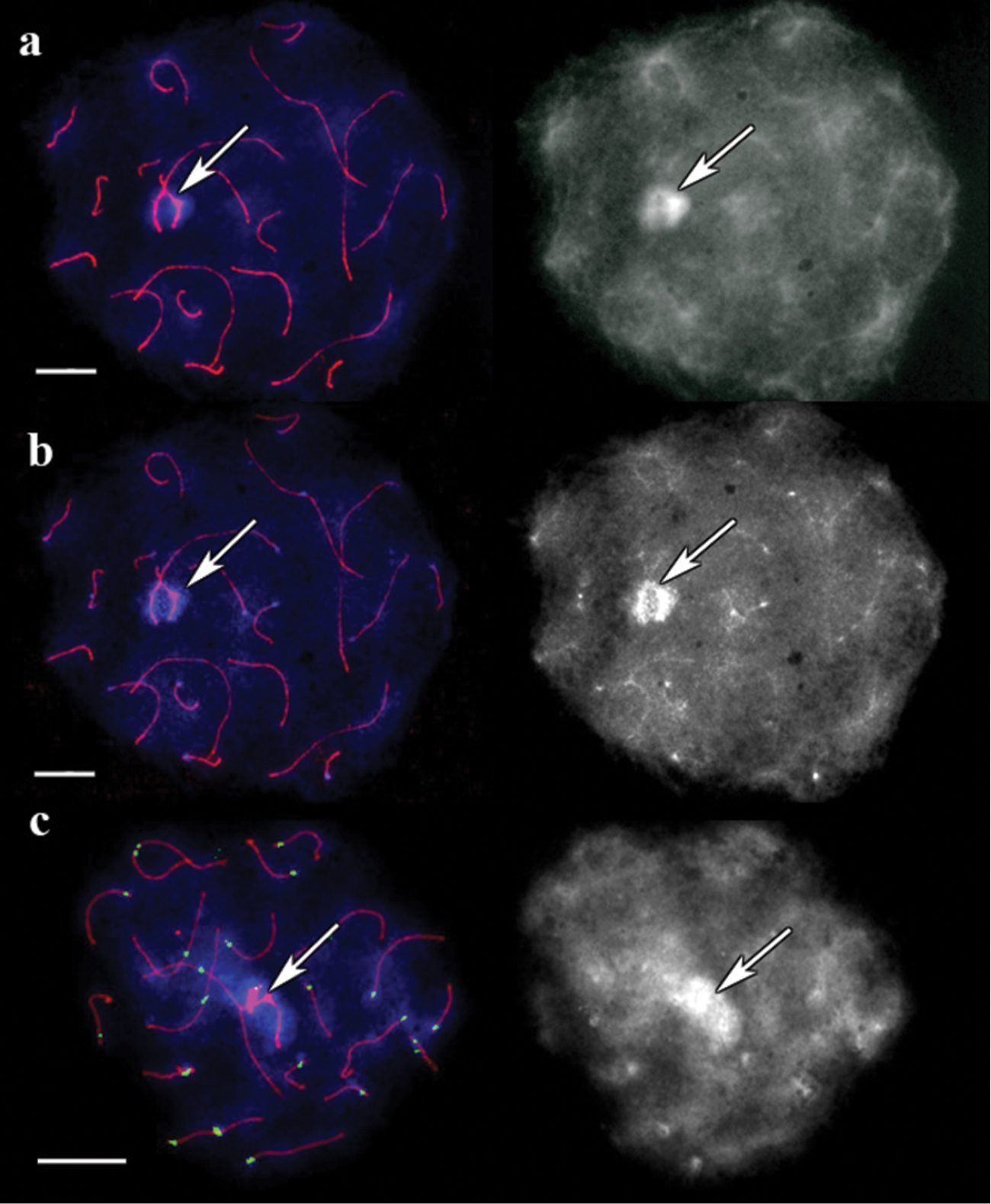
In pigmy shrew the DAPI-stained pachytene chromosomes untreated with barium hydroxide produced a unique pattern for each bivalent. This result agrees with that of (
SC spread in red-backed vole. a A cell after immunostaining, prior to barium hydroxide treatment. Left: Red shows SCs (long lines) and centromeres (dots), blue represents DAPI. Right: the same image, DAPI channel separately b The same cell after 5 min of barium hydroxide treatment. Left: Red shows to SCs (long lines) and centromeres (dots), blue represents DAPI. Right: the same image, DAPI channel separately. Arrows indicate sex bivalent. Scale bars = 20 μm.
SC spread in guinea pig. a A cell after immunostaining, prior to barium hydroxide treatment. Left: Red, green and blue indicate SCs, centromeres, and DAPI, respectively. Right: the same image, DAPI channel separately b The same cell after 5 minutes of barium hydroxide treatment. Left: Red, green and blue indicate SCs, centromeres, and DAPI, respectively. Right: the same image, DAPI channel separately. Arrows indicate sex bivalent. Scale bars = 20 μm.
SC spread in pigmy shrew. a A cell after immunostaining, prior to barium hydroxide treatment. Left: Red shows SCs, and blue represents DAPI. Right: the same image, DAPI channel separately b The same cell after 5 minutes of barium hydroxide treatment. Left: Red shows SCs, and blue represents DAPI. Right: the same image, DAPI channel separately c Bright DAPI spots with their corresponding centromere signals. The cell after 5 min of barium hydroxide treatment. Left: Red, green and blue indicate SCs, centromeres, and DAPI, respectively. Right: the same image, DAPI channel separately. Arrows indicate sex bivalent. Scale bars = 20 µm.
In domestic cat (Felis silvestris catus (Linnaeus, 1758)) and red fox (Vulpes vulpes (Linnaeus, 1758)), the centromeres were successfully detected by ACA, but any centromere-specific DAPI staining was not observed on either untreated pachytene chromosomes, or barium hydroxide-treated chromosomes. The results were not improved although the treatments were modified by increasing HCl pre-incubation time to 30 min, extending barium hydroxide incubation time to 10 min, or decreasing barium hydroxide incubation time to 1 min. The failure to obtain centromeric DAPI signal on the pachytene chromosomes of domestic cat and red fox corresponds to previously published data about the absence of centromeric C-bands and centromeric satellite DNA in cat chromosomes (
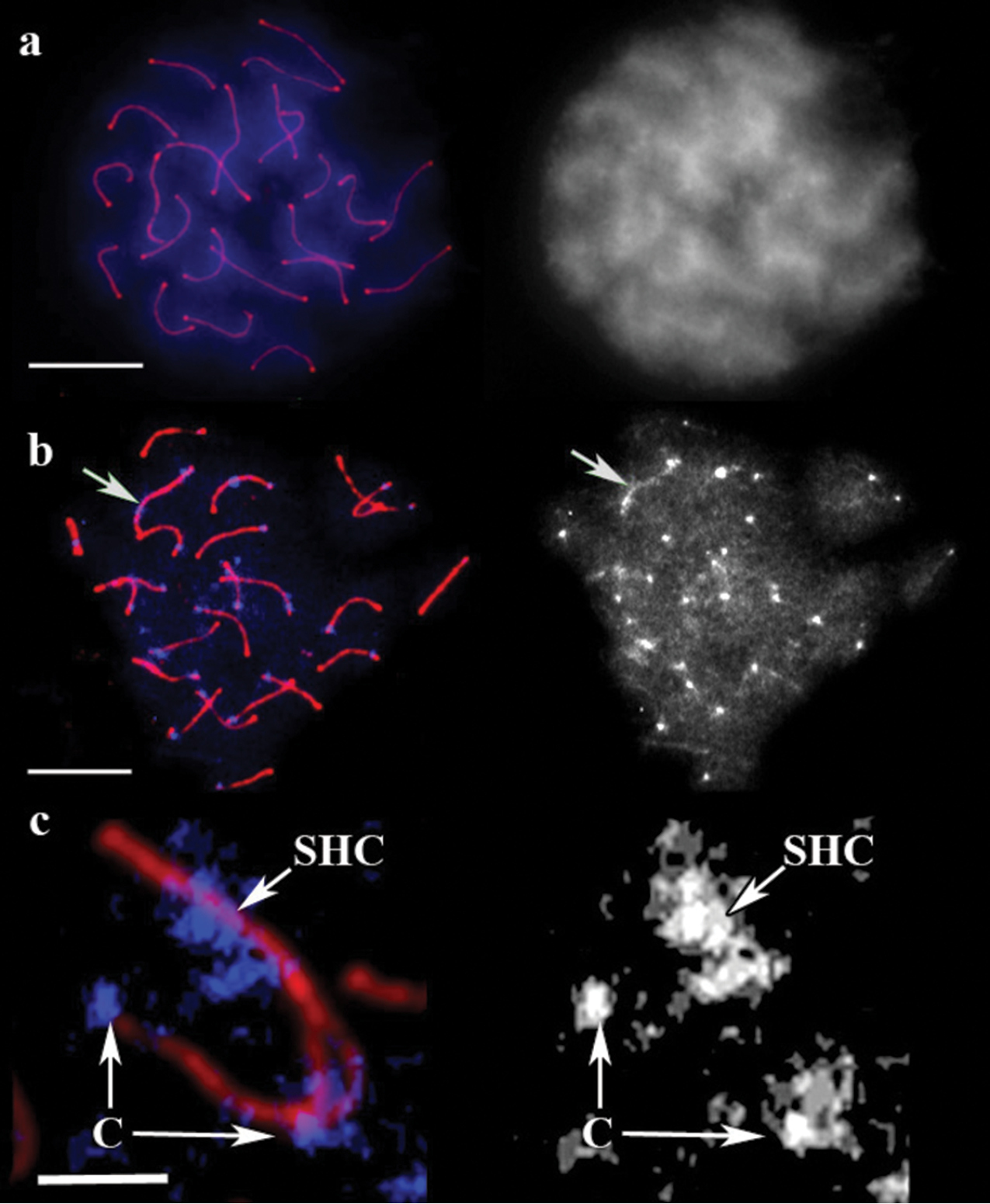
DAPI staining of untreated guppy male pachytene chromosomes produced completely uniform fluorescence without any specific signals (Fig. 4a). ACA did not also show any specific signal in the cell, although the concentration was five times higher than that recommended by the manufacturer. After 5 min of barium hydroxide treatment a bright spot appeared at the end of each bivalent, and the residual fluorescence faded (Fig. 4b). DAPI spots were mostly located at SC ends opposite to the recombination nodules. This finding was attributed to suppressed recombination near the centromeres. This effect has also been described in various vertebrate species including mammals (
The XY bivalent in guppy was easily distinguishable from a delayed synapsis at early and mid pachytene stages and from an excessive thickening of the axial elements at late pachytene stage. This bivalent showed one small DAPI signal at one end and a large DAPI-positive block close to its opposite end (Fig. 4c). This result is consistent with the pattern obtained by (
In guppy, staining quality depend on the age of preparations. In this study, optimal results were obtained from two to six month old preparations. Centromeric and sex fluorescent blocks were also visible on fresh slides, but only after image brightness and contrast were adjusted. Although the time allotted for barium hydroxide treatment was extended to 10 min, optimal results from the fresh preparations were not obtained. This may occur possibly because higher level of DNA degradation in the old preparations facilitated denaturation by barium hydroxide.
SC spread in guppy. a A cell after immunostaining, prior to barium hydroxide treatment. Left: Red shows SCs, and blue represents DAPI. Right: the same image, DAPI channel separately. Scale bar = 20 μm. b The cell after 5 min of barium hydroxide treatment. Left: Red shows SCs, and blue represents DAPI. Right: the same image, DAPI channel separately. Arrow indicates sex bivalent. Scale bar = 20 μm. c Sex bivalent during pairing. Red shows SC, blue represents DAPI. C: centromere, SHC: sex heterochromatin. Scale bar = 5 μm.