(C) 2013 Ana Cláudia Swarça. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Hemisorubim platyrhynchos is a medium- to large-sized pimelodid catfish distributed along several river basins of the Neotropical Region, noteworthy for representing an important fishery source. In this work, Hemisorubim platyrhynchos from three isolated populations were cytogenetically analyzed. The karyotype shows a diploid number of 2n=56 chromosomes comprising 22m, 16sm, 10st, 8a (FN=104). NORs detected by AgNO3 were located in the terminal regions of the short arm of a st chromosome pair, as confirmed by CMA3 and FISH using an 18S rDNA probe. C-banding revealed a small amount of heterochromatin in chromosomes, including the NORs, and one biarmed pair that showed conspicuous positive bands on both arms. This fact was also evidenced when using other banding techniques, such as RE (AluI), and indicates that this pair constitutes a species-specific cytogenetic marker.
Hemisorubim platyrhynchos, Pimelodidae, Cytogenetics
Hemisorubim platyrhynchos (Valenciennes, 1840), popularly called “jurupoca” or porthole shovelnose catfish, is a pimelodid fish inhabiting the deeper and slow-moving sections of large South American rivers (
The family Pimelodidae represents one of the most specious catfish groups, however relationships among species of this group still remain as unanswered questions; however, it seems self-evident that they share certain characteristics (Nelson 2006,
From a cytogenetic point of view some reports show that these groups could also share cytogenetic characteristics, supporting additionally the classification above mentioned (
Hemisorubim platyrhynchos is a monotypic species that belongs to the family Pimelodidae, however, it is considered one of the “sorubimine catfishes”, an informal group of catfish that comprises other genera such as Sorubim Cuvier, 1829, Pseudoplatystoma Bleeker, 1862, and Brachyplatystoma Bleeker, 1862 (
Until now only one population of Hemisorubim platyrhynchos of the Parana River (Brazil) has been cytogenetically studied and has had its diploid number, AgNORs location and C-banding reported (
The objective of the present study was to describe the karyotypic structure of specimens from three populations of Hemisorubim platyrhynchos aiming to characterize andcompare the obtained results with the available cytogenetic data on this and other related species.
Fifteen specimens of Hemisorubim platyrhynchos consisting of 8 males (m) and 5 females (f), caught in the Parana River (Corrientes State, Argentina) and 2 specimens of undetermined sex from the Miranda River (Mato Grosso do Sul State, Brazil) were cytogenetically analyzed. The sampling sites in the Paraná River were: Ituzaingó (2 m), Itá Ibaté (2 f - 3 m), Yahapé (1 m), Puerto Abra (1 f), and Corrientes (2 f / 2 m) (Corrientes Province). Mitotic chromosome preparations were obtained according to the technique described by
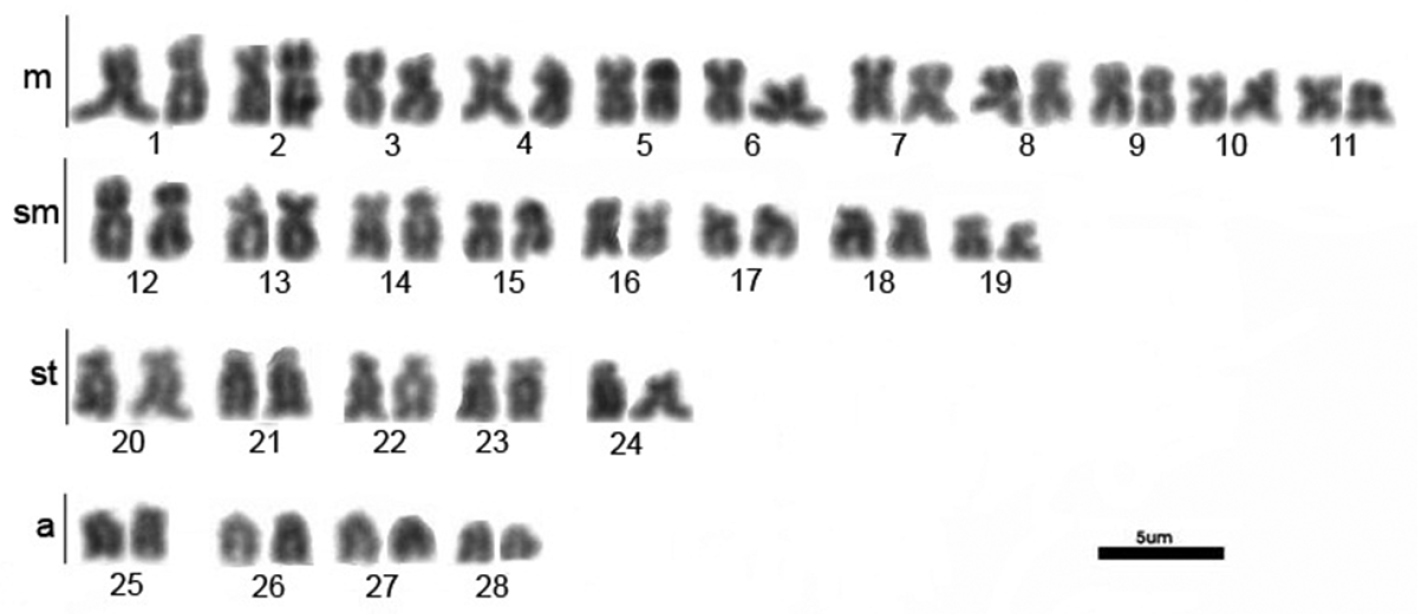
All three populations of Hemisorubim platyrhynchos presented the same results. The diploid number obtained was 2n=56 and the karyotype was composed of 22m+16sm+10st+8a (NF=104) without chromosomal differences between sexes (Fig. 1).
Karyotype of Hemisorubim platyrhynchos. Bar = 5mm.
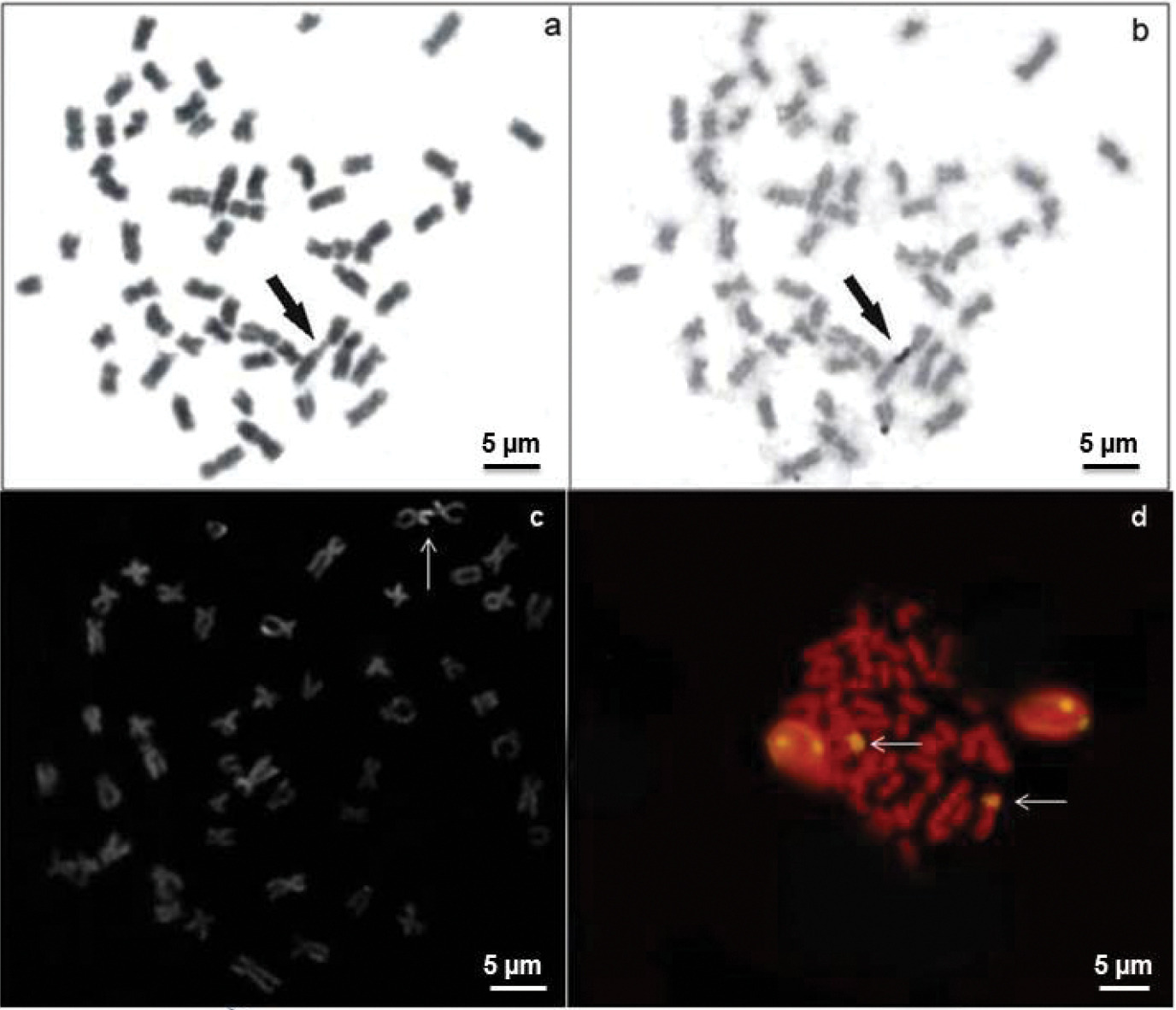
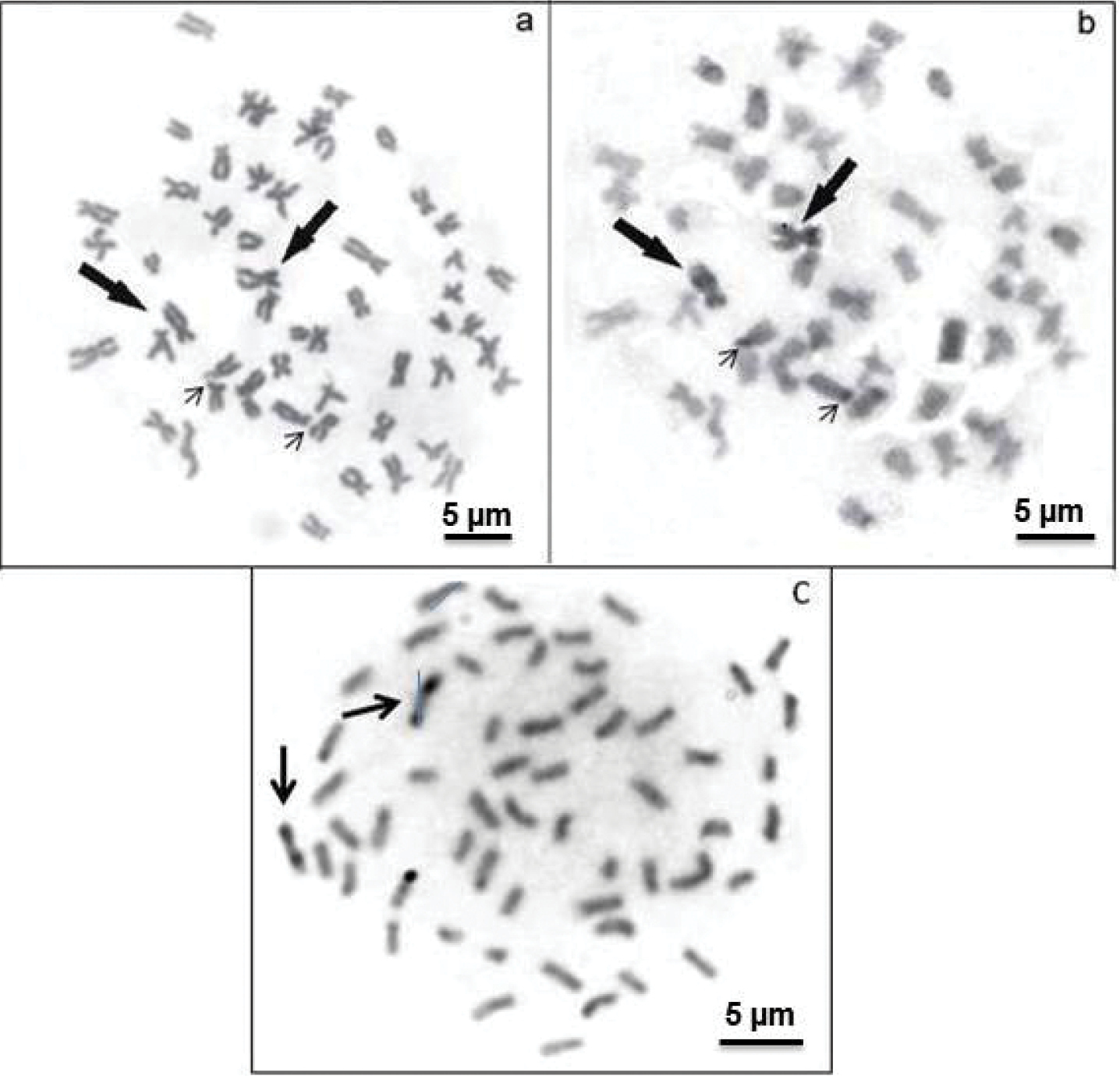
The AgNORs were located in the terminal position on the short arm of a subtelocentric (st) pair (Figs 2a, b). The bright signals correspond to the zones evidenced by argentic impregnation after FISH with the 18S rDNA probe and staining with CMA3 (Figs 2c, d). This chromosome pair is clearly identified due to its size, shape and exclusive secondary constriction. C-banding revealed positive bands in the pericentromeric regions of some chromosome pairs and on the short arms of a st chromosome pair, coincident with positive C-bands and allowed the identification of a large biarmed marker pair with positive bands on almost the entire short and long arms (Fig. 3b). The AluI restriction enzyme shows bands that resemble C-banding, principally on the biarmed chromosome, producing a reverse pattern (Fig. 3c). The mentionedchromosome could be considered a species-specific cytogenetic marker, since it has not been reported in other species of this group of fish.
Metaphases of Hemisorubim platyrhynchos showing sequential Giemsa-AgNO3 staining (a, b) CMA3 banding (c) FISH with 18S rDNA probe (d). Arrows indicate the NOR- bearing chromosomes.
Metaphases of Hemisorubim platyrhynchos showing sequential Giemsa-C banding (a, b) and after AluI treatment (c). The arrows indicate the biarmed chromosome pair (marker) with positive bands on the short and long arms, the thin arrows show the NOR-bearing chromosomes.
The karyotype of Hemisorubim platyrhynchos was composed of 22m+16sm+10st+8a (NF=104), however, despite having the same diploid chromosome number 2n=56, Hemisorubim platyrhynchos from the Paraná River/Brazil reported by
One point worth emphasizing is the homogeneity of the karyotypes of species belonging to the “Sorubiminae group” with a clear prevalence of biarmed chromosomes, showing a high fundamental number. A cytogenetic feature shared by all species of this group is the AgNORs localized in the terminal position on the short arm of one pair of st/a chromosomes that also could be evidenced by C-banding, as observed in the present study and in other studied species, such as Sorubim lima (Bloch & Schneider, 1801)(
CMA3 staining and FISH with 18S rDNA exhibited fluorescent signals that correspond to the AgNOR sites (Fig. 2c, d). This correspondence between AgNORs, C-banding, FISH and CMA3 staining has already been observed in almost all species of the Pimelodidae family (
The relatively low amount of heterochromatin in chromosomes of Hemisorubim platyrhynchos andin other species of the Pimelodidae catfishes suggests that this may be a characteristic of this family. On the other hand, C-banding allowed the identification of a large biarmed pair with positive bands on almost the entire short arm and on the long arm. The AluI restriction enzyme onfish chromosomes produces a C-banding-like pattern (
According to cytogenetic traits, this family could be divided into two: the “Pimelodus group” and the “Sorubiminae group” (=Sorubinae), and the cytogenetic data confirm that the analyzed species belongs to the second group, because it has 2n=56 chromosomes, a high NF and the NORs localized on one single chromosome pair in the terminal position of the short arms, as it occurs with the other species of this group (