(C) 2013 Christina Nokkala. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Nokkala C, Kuznetsova VG, Nokkala S (2013) Meiosis in rare males in parthenogenetic Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) populations from northern Europe. Comparative Cytogenetics 7(3): 241–251. doi: 10.3897/CompCytogen.v7i3.6126
For studying meiosis in males, large samples of Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) were collected in Norway, Sweden, Finland and northwest Russia. In addition to all-female populations, males were present in 10 out of 47 populations; still, all populations were highly female-biased, the proportion of males varying from 0.1% to 9.1%. These males are thus rare or so-called spanandric males. Males in northern Norway, Finland and northwest Russia showed normal chiasmate meiosis, while complete absence of chiasmata due to asynapsis was found in males collected in Norway and northern Sweden. In asynaptic meiosis, all univalent chromosomes divided during the first meiotic division resulting in incomplete second meiotic division and formation of diploid sperms. Hence, males in these populations are nonfunctional and do not contribute to the genetic constitution of the population, but appear in every generation as reversals from apomictic parthenogenesis and the mode of parthenogenesis is of obligatory type.
Cacopsylla myrtilli, parthenogenesis, spanandric males, asynaptic meiosis, nonfunctional males
For bisexual reproduction it is essential that the diploid chromosome complement is reduced to haploid during gametogenesis. This is achieved by a specialized cell cycle, meiosis, where the chromosome number of germ line cells is reduced during two rounds of cell divisions after just one round of chromosome replication. In the first, reductional division homologous chromosomes segregate from each other while in the other, equational division individual chromosomes divide. During early prophase of the first meiotic division, homologous chromosomes pair, undergo tight synapsis and form a bivalent. Bivalent integrity is retained throughout prophase with either of two ways. Most often a crossing-over occurs during synapsis creating a physical link or chiasma between the homologs, which are held together until anaphase I. Alternatively, in achiasmate meiosis, pairing, alignment and synapsis are normal, but no chiasmata are formed. In these cases, the paired condition of homologs is retained till the onset of anaphase I (for references see
In natural populations, the meiotic mechanism is under strong selection; all mutations causing disturbances in meiosis are quickly removed from the population. The only exceptions are thelytokous parthenogenetic populations, where males are occasionally found (
The object of our study is the Holarctic psyllid species Cacopsylla myrtilli (Wagner, 1947), which has long been considered as parthenogenetic (
For the present study we have collected Cacopsylla myrtilli from many populations from Norway including recollection of
Adult Cacopsylla myrtilli specimens were collected in Norway, Sweden, Finland and northwest Russia. For the present study, we selected only those populations, where males were found (Table 1). The Rindhovda population in Norway has been earlier studied by
Specimens were fixed immediately after collection in the field in freshly made 3:1 Carnoy and stored in fixative in the laboratory at + 6°C until slides were made according to the method by
Locations of Cacopsylla myrtilli populations in Norway, Sweden, Finland and Russia. Number of cytologically studied males, total number of males, number of females and frequency of males.
| Population | latitude, longitude | altitude | males | females | male freq. | ||
|---|---|---|---|---|---|---|---|
| studied | total | ||||||
| Norway | |||||||
| Finnmark, Šuoššjávri | 69°22'11"N, 24°18'20"E | 3 | 4 | 2950 | 0.001 | ||
| Sjoa, Rudihøe | 61°46'27"N, 9°17'16"E | 1000 | 1 | 3 | 1078 | 0.003 | |
| Sjoa, Kvernbrusætrin | 61°42'27"N, 9°19'25"E | 950 | 15 | 22 | 1443 | 0.015 | |
| Sjoa, Stålane | 61°41'15"N, 9°14'27"E | 1000 | 11 | 14 | 470 | 0.030 | |
| Sjoa, Kringlothaugen | 61°43'06"N, 9°22'40"E | 700 | 1 | 1 | 277 | 0.004 | |
| Sjoa, Rindhovda | 61°43'05"N, 9°05'12"E | 1080 | 30 | 38 | 379 | 0.091 | |
| Sweden | |||||||
| Abisko, Lapporten | 68°19'26"N, 18°51'05"E | 610 | 5 | 5 | 386 | 0.013 | |
| Finland | |||||||
| Utsjoki | 69°51'06"N, 27°00'34"E | 2 | 3 | 2603 | 0.001 | ||
| Paltamo | 64°33'28"N, 27°43'41"E | 6 | 10 | 1595 | 0.006 | ||
| Russia | |||||||
| White Sea, Sredny Island |
66°17'00"N, 33°40'00"E | 9 | 49 | 2946 | 0.016 | ||
†Data from
For staining of chromosomes the Feulgen-Giemsa method by
Chromosomes were photographed with BU4-500C CCD camera (BestScope International Limited, Beijing, China) mounted on Olympus BX51 microscope (Olympus, Japan) using ISCapture Sofware version 2.6 (Xintu Photonics Co LTD, Xintu, China). Photographs were processed with Corel Photo-Paint X5 software.
We found males in ten (Table 1) out of a total of 47 populations collected. All populations studied were highly female-biased, male frequency varying from 0.1 % to 9.1 %. The highest proportion of males was found from Rinhovda population in Norway collected above tree line. This same population was earlier studied by
The testes in male psyllids are organized into lobed structures, the number of which varies in a species specific manner (
Males collected in Finnmark, Šuoššjávri in Norway, in Paltamo and Utsjoki in Finland and the White Sea area in Russia (
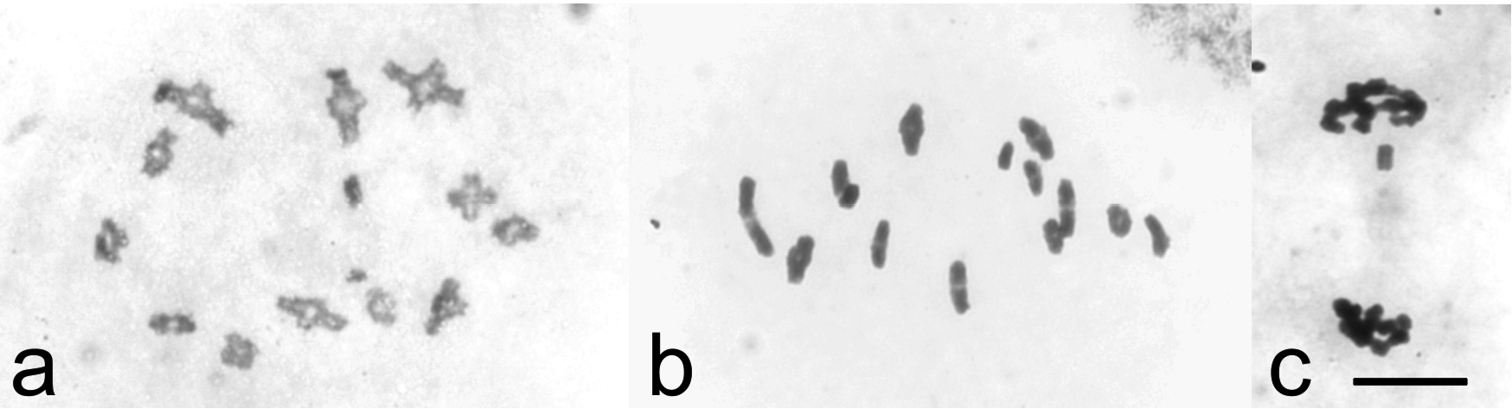
Males from northern Norway, Finland and Russia showed meiotic stages typical for psyllids known to possess holocentric chromosomes. The most common stage in testes was the diffuse stage during which chromatin had a diffuse appearance covering the whole nucleus in a cell. During this stage the size of cells is increased considerably. When the chromosomes condensed out from the diffuse stage, diplotene stage with 12 autosomal bivalents with one chiasma in each and a univalent X chromosome were seen (Fig. 1a). At metaphase I, the bivalents showed axial orientation with homologous telomeres oriented to opposite poles and the univalent X chromosome lying at the equatorial plane (Fig. 1b). Segregation of bivalents at anaphase I resulted in half-bivalents moving towards the poles. The bi-oriented X chromosome was seen as a laggard at this stage. At telophase I, the X chromosome moved to one of the poles (Fig. 1c), resulting in two kinds of secondary spermatocytes, those with the X chromosome and those without it.
Chiasmate male meiosis in Cacopsylla myrtilli (a) diakinesis with twelve autosomal bivalents an univalent X chromosome (b) metaphase I with twelve chiasmate bivalents and univalent X (c) telophase I, univalent X chromosome moving towards upper pole. Bar equals 10 µm.
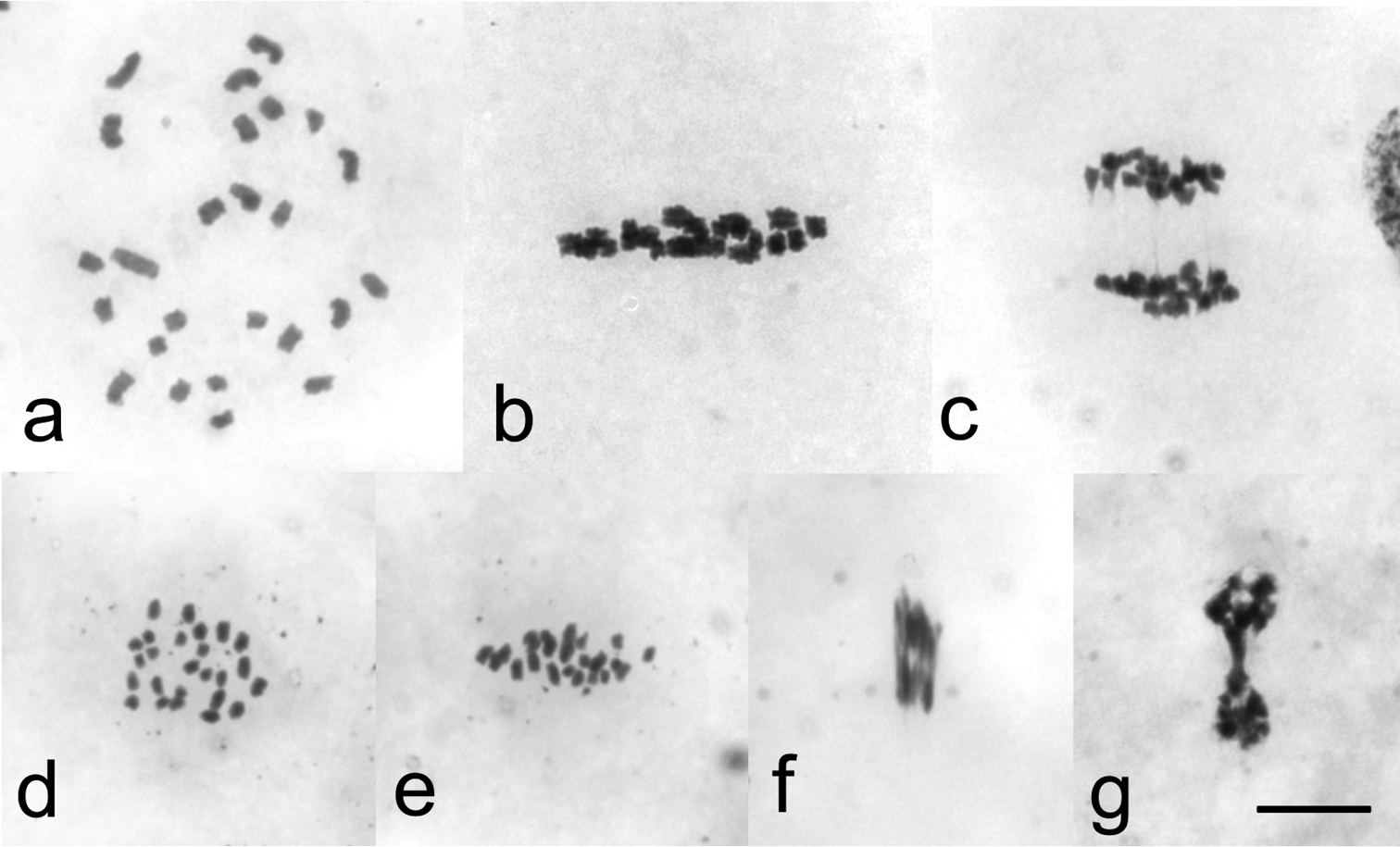
However, males collected near Sjoa, Norway (Rindhovda, Rudihøe, Kvernbrusætrin and Stålane) and northern Sweden (Abisko, Lapporten) showed 25 (24 + X) univalent chromosomes at diplotene and diakinesis stages, indicating a complete failure in chiasma formation (Fig. 2a). Univalent chromosomes oriented with sister chromatids to opposite poles at metaphase I (Fig. 2b). These bi-oriented chromosomes divided at anaphase I. No laggard was seen at anaphase I, indicating that the X chromosome also divided in the first meiotic division (Fig. 2c). Anaphase I resulted in secondary spermatocytes with 25 daughter chromosomes, oriented to both poles at metaphase II (Fig. 2d, e). As daughter chromosomes are unable to divide, anaphase II started but could not be completed (Fig. 2f, g). Consequently, diploid spermatids were produced. Meiosis resulted in a cyst of 128 developing spermatids instead of the 256 in normal chiasmate meiosis.
Male meiosis without chiasmata in Cacopsylla myrtilli (a) Diakinesis with 25 univalent chromosomes (b) Metaphase I in side view with 25 bi-oriented univalents (c) Anaphase I, daughter chromosomes moving towards opposite poles. No laggard chromosomes present (d) Metaphase II in polar view showing 25 daughter chromosomes (e) Metaphase II in side view. All bi-oriented daughter chromosomes aligned with the equatorial plane (f) Anaphase II, daughter chromosomes stretched towards poles (g) Telophase II, daughter nuclei joined by stretched chromosomes. Bar equals 10 µm.
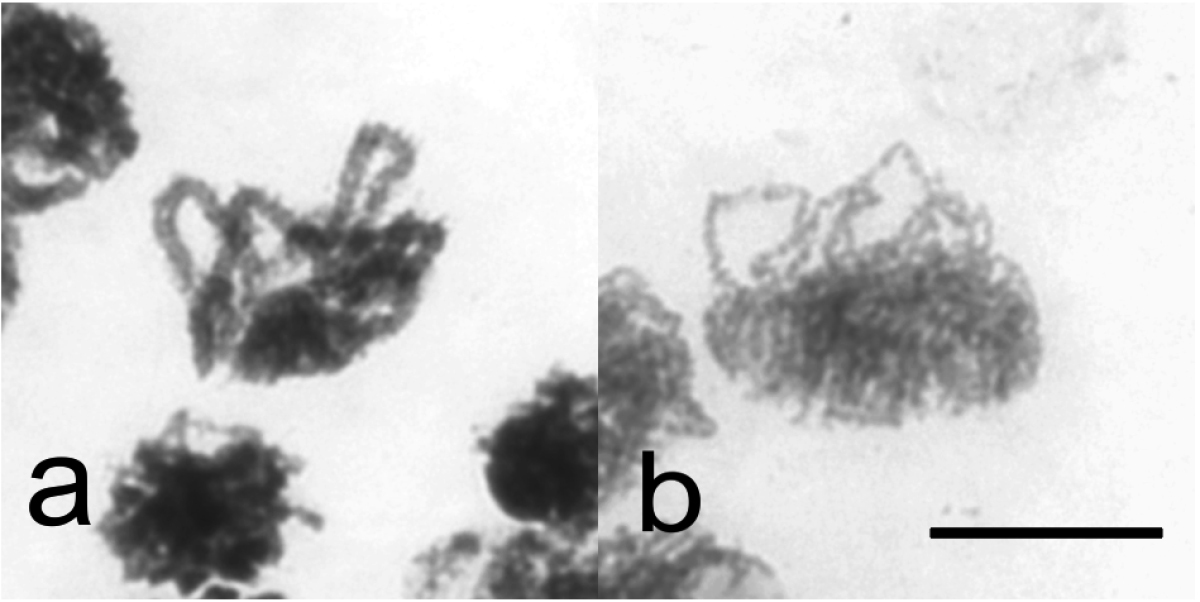
To find out reasons for the absence of chiasmata, early stages of meiosis were analysed in more detail. In chiasmate male meiosis pachytene stage (Fig. 3a) was easily found in all males studied. However, in males without chiasmata no pachytene cells were found in 61 males studied, but leptotene-like nuclei were abundant. When seen in side-view chromosomes showed distinct bouquet orientation (Fig. 3b), indicating that presynaptic alignment was normal, but synapsis did not occur. It seems apparent then that the failure in chiasma formation in these males is due to a mutation resulting in asynapsis of homologous chromosomes.
Synapsis and synaptic alignment of chromosomes in Cacopsylla myrtilli (a) Pachytene, synapsed chromosomes in bouquet orientation in chiasmate male meiosis (b) Presynaptic alignment of leptotene chromosomes in male meiosis lacking chiasmata in bouquet orientation. Bar equals 10 µm.
In the present study we have established that none of the male-carrying populations in Norway, northern Sweden, Finland and northwest Russia were genuinely bisexual, but all were parthenogenetic with highly female-biased sex ratio. Variation of male frequencies from 0.1 to 9.1 % was quite similar to that previously found in parthenogenetic oribatid mites, in which frequencies are varying from 0.15 % to 6.3 % (
Although complete failure in chiasma formation in male meiosis was found for the first time in a natural population in the present study, the phenomenon is well known in the holocentric laboratory model organism, the nematode Caenorhabditis elegans (Maups, 1900). Genetic dissection studies on early events in meiosis, presynaptic alignment, formation of double strand breaks, synapsis and crossing over, and chiasma formation, have revealed several gene loci, in which mutations result in complete lack of crossing over in an affected animal (for reviews see
Both mitosis and meiosis include surveillance mechanisms to ensure regular behavior of chromosomes. In meiosis, homologous chromosomes pair and align with each other followed by their tight synapsis that allows exchange process or crossing over between homologs necessary for maintaining bivalent configuration later in meiotic prophase. These early stages are controlled by the pachytene checkpoint. Defects in synapsis or recombination result in meiotic arrest or apoptosis, thus preventing the formation of defective gametes (
The orientation of chromosomes in metaphase spindle is monitored by the spindle assembly checkpoint. As far as even one chromosome does not reach a proper spindle microtubule attachment to the spindle pole, the onset of anaphase in mitosis or anaphase I or anaphase II in meiosis is inhibited. Once all the chromosomes display proper orientation, the onset of anaphase is triggered by anaphase promoting complex that activates separase enzyme that will degrade sister chromatid cohesion (
In meiosis, there are mechanisms, which are responsible for the regular segregation of univalent chromosomes, especially in the case of specialized chromosomes like sex chromosomes (
In this study we have established that males occurred in ten out of 47 Cacopsylla myrtilli populations sampled in northern Europe. All populations were highly female-biased, the proportion of males varying from 0.1 % to 9.1 %. Thus, all populations are in fact parthenogenetic and males are rare or so-called spanandric males. In northern Norway, Finland and Russia male meiosis was normal whereas in Norway and Sweden males displayed asynaptic meiosis leading to the formation of diploid spermatids. These males are nonfunctional in reproduction and appear in every generation as reversals from apomictic parthenogenesis.
P. Strelkov, G. Paskerova and O. Gotenko are acknowledged for collecting material from northwest Russia. This work was supported by the University of Turku Foundation project grant to SN and CN and by the Russian Foundation for Basic Research (grant 11-04-00787) and the program of the Presidium of RAS "Origin of the Biosphere and Evolution of Geo-biological Systems" to VK.