(C) 2013 Bo Xu. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Xu B, Li YK, Hua BZ (2013) A chromosomal investigation of four species of Chinese Panorpidae (Insecta, Mecoptera). Comparative Cytogenetics 7(3): 229–239. doi: 10.3897/CompCytogen.v7i3.5500
The male adults of four species of the Chinese Panorpidae in Mecoptera were cytogenetically studied using conventional squashing procedures. The results show that their sex-chromosome system belongs to the XO type, with n = 19 + X(O) in Panorpa emarginata Cheng, 1949 and Panorpa dubia Chou & Wang, 1981, n = 23 + X(O) in Panorpa sp., and n = 20 + X(O) in Neopanorpa lui Chou & Ran, 1981. X chromosomes of these species usually appear dot-shaped in late prophase I and are easily differentiated from autosomal bivalents. Meiosis in these Panorpidae lacks typical diplotene and diakinesis. In late prophase I, pairs of homologous chromosomes remain parallel in a line and show no evidence of crossing-over. Some of them even appear as a single unit because of extremely intimate association, all with a tendency of increasing condensation. The evolutionary significance of their chromosomal differences and the achiasmatic meiosis of Panorpidae are briefly discussed.
Mecoptera, Panorpidae, chromosome, XO sex-chromosome system, achiasmatic male meiosis
Mecoptera are one of the minor orders of holometabolous insects with approximately 650 described species worldwide (
Panorpidae are the most species-rich family in Mecoptera, with over 420 described species assigned to six genera (
The cytogenetics of Mecoptera received a passing interest from the 1930s to the 1970s. To date, only some European and American species have been cytogenetically studied. Species of Panorpa were first reported to have an XO sex determination mechanism in males and to have a fairly high complement number (more than 40) by
To increase our knowledge of the cytogenetic nature and chromosomal evolution in Mecoptera, we studied meiosis in four species of the Chinese Panorpidae, including three species of Panorpa and one more species of Neopanorpa.
Male adults of Panorpa emarginata Cheng, 1949, Panorpa dubia Chou & Wang, 1981, Panorpa sp., and Neopanorpa lui Chou & Ran, 1981 were investigated using conventional squashing procedures. At least three specimens of each species were sampled. The examined species and their localities are listed in Table 1.
The examined species and their localities.
| Species | Localities | Collection date |
|---|---|---|
| Panorpa emarginata | Taibai Mountain, Shaanxi | Early June 2007 |
| Panorpa dubia | Huoditang Forest Farm, Shaanxi | Early June 2012 |
| Panorpa sp. | Tongbai Mountains, Henan | Late July 2012 |
| Neopanorpa lui | Nangong Mountain, Shaanxi | Middle June 2012 |
Testes of these species were extracted from ethyl ether anaesthetized specimens and subjected to hypotonic treatment in 0.48% solution of potassium chloride for 15 min, then fixed in a mixture of methanol and acetic acid (3:1) for 2 h. The fixed testes were squashed and stained with 1% Giemsa in Sörense buffer solution (0.067 mol/L, pH 6.8) for 10 min except for Panorpa emarginata, which was stained with 2% hematoxylin solution for 10 sec. Photographs were taken with a Nikon DS-Fil digital camera equipped with a Nikon Eclipse 80i microscope.
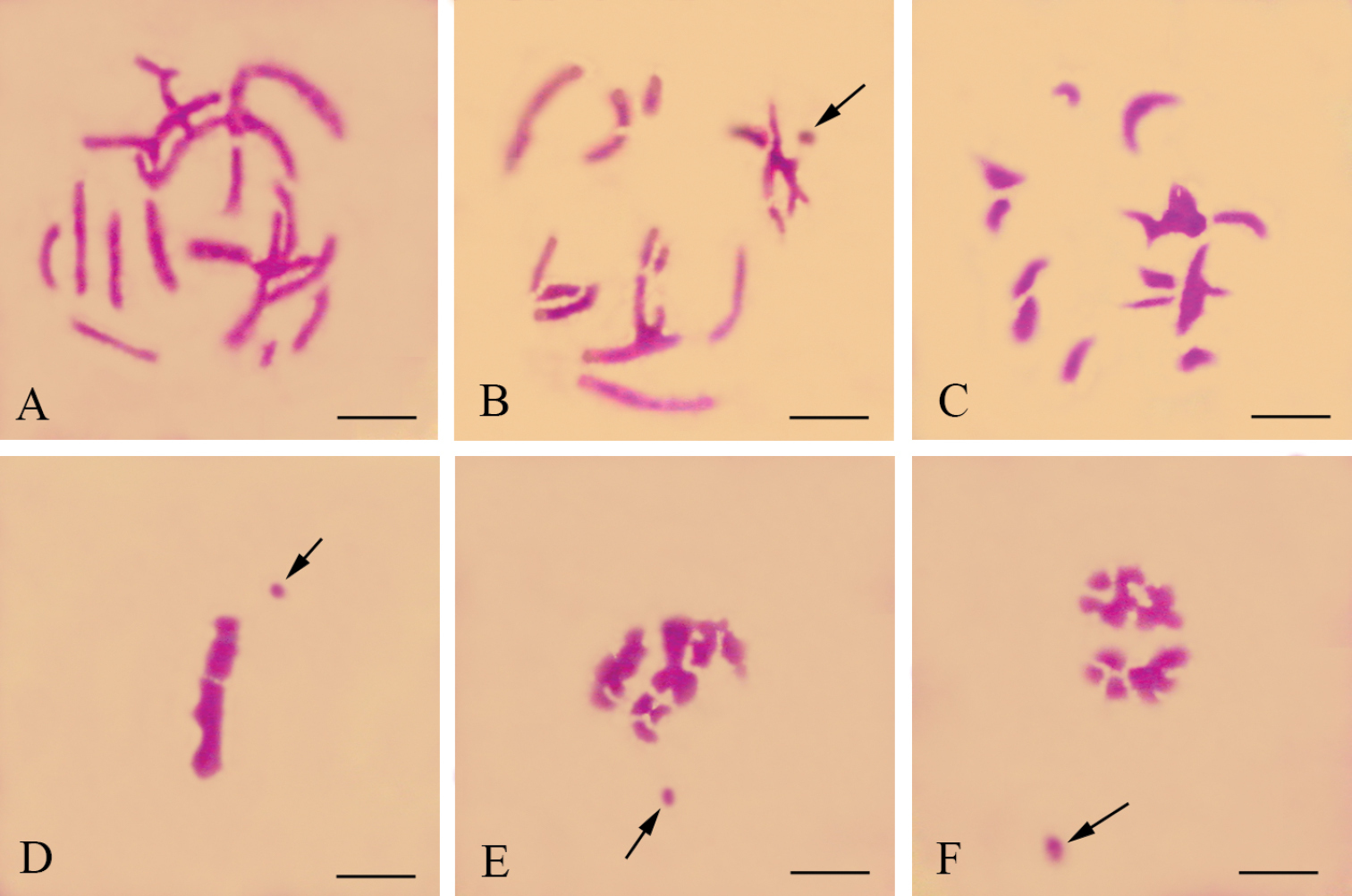
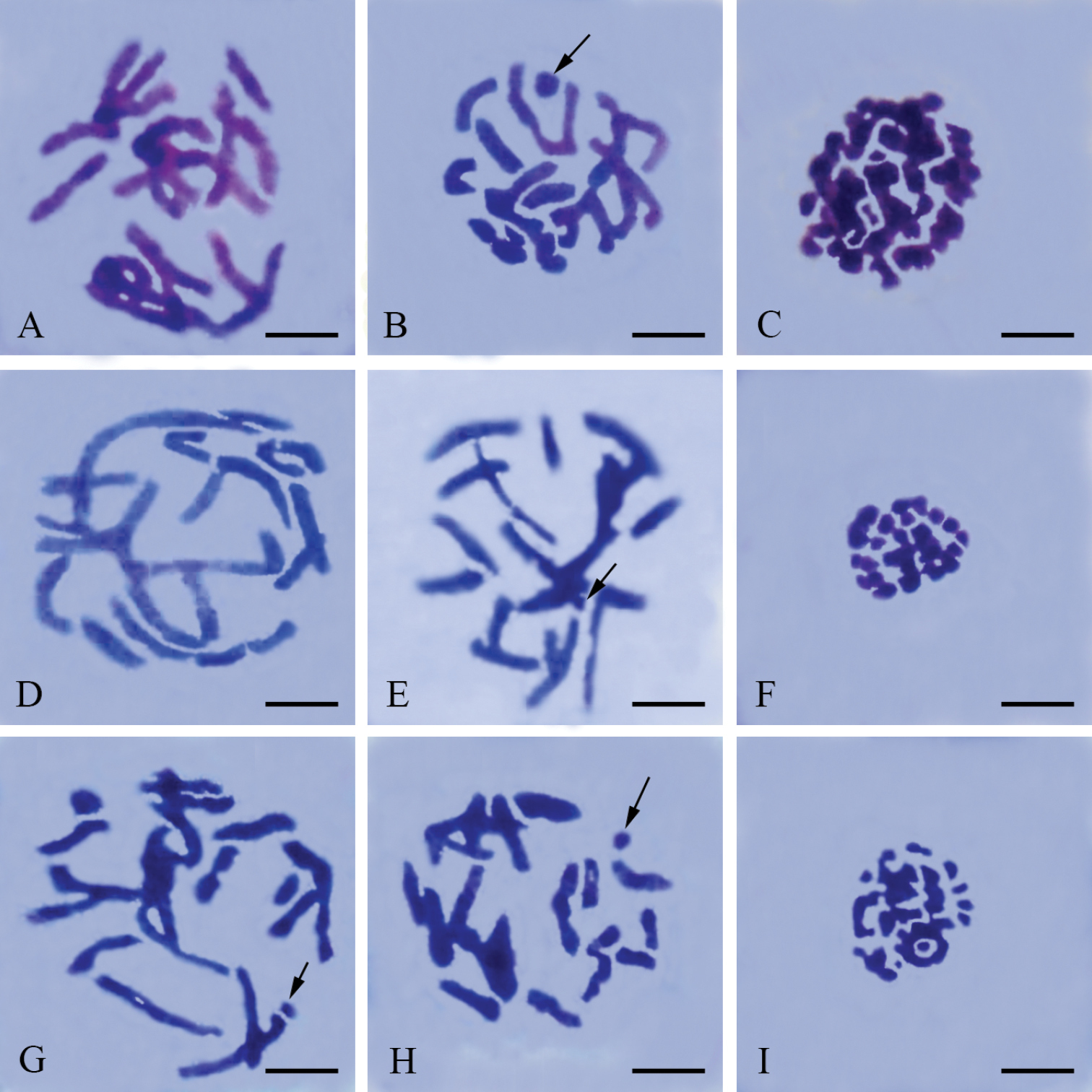
The males have a meioformula of n = 19 + X(O) (Fig. 3A). In pachytene (Fig. 1A), the X univalent and autosomal bivalents are all strip-shaped. It is difficult to distinguish the X univalent from autosomal bivalents. In late prophase I (Fig. 1B), the X univalent is dot-shaped and the homologous chromosomes of each bivalent are closely associated. No traces of crossing-over were observed. In pre-metaphase (Fig. 1C), the chromosomes become much more condensed. In the lateral view of metaphase I (Fig. 1D), all bivalents are located in the equatorial plate with the X univalent being precocious. In the lateral view of anaphase I (Figs 1E, 1F), the X can also move ahead of other bivalents.
Meiotic chromosomes of male Panorpa emarginata subjected to hematoxylin staining. A pachytene B late prophase I showing the dot-shaped X univalent (arrow) and achiasmatic bivalents C metaphase I showing substantially more condensed chromosomes D lateral view of metaphase I showing congression of autosomal bivalents and precocity of X univalent (arrow) E, F lateral view of anaphase I showing precocity of X univalent (arrow). Bars = 10 μm.
The males also have a meioformula of n = 19 + X(O) (Fig. 3B). In pachytene (Fig. 2A) these bivalents are rod-shaped and almost of the same size, but the X univalent is difficult to observe. In late prophase I (Fig. 2B), the bivalents become more condensed and the X univalent appears dot-shaped. As in Panorpa emarginata, homologous chromosomes are associated with each other so intimately that they appear as single units. No indication of crossing-over was observed. In the polar view of metaphase I (Fig. 2C), the majority of these bivalents present parallel-arranged homologous chromosomes.
The males have a meioformula of n = 23 + X(O) (Fig. 3C). In pachytene (Fig. 2D) the X univalent is difficult to observe. In late prophase I (Fig. 2E), these bivalents become condensed and the X univalent is usually dot-shaped. In particular, some bivalents exhibit two parallel-arranged homologous chromosomes without chiasmata. In the polar view of metaphase I, only a few bivalents present parallel-arranged homologous chromosomes (Fig. 2F).
Meiotic chromosomes of male Panorpidae. A–C Panorpa dubia D–F Panorpa sp. and G–I Neopanorpa lui subjected to Giemsa staining A, D pachytene B, E, G, H late prophase I showing more condensed bivalents than in pachytene and the dot-shaped X univalent (arrow). C, F, I polar view of metaphase I. Bars = 5 μm.
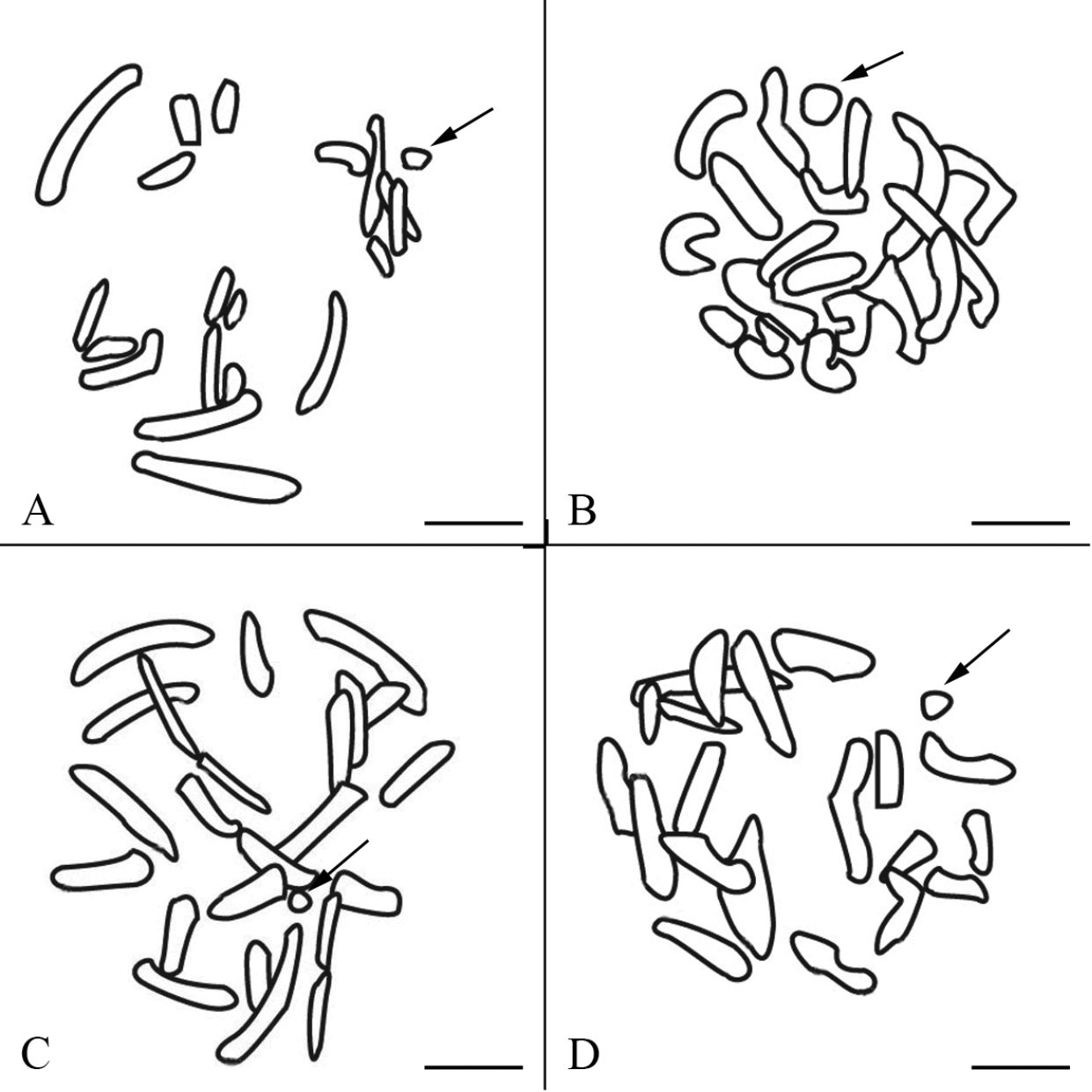
Schematic drawings of late prophase I chromosomes. A Panorpa emarginata B Panorpa dubia C Panorpa sp. and D Neopanorpa lui corresponding to Fig. 1B, Fig. 2B, Fig. 2E and Fig. 2H, respectively. The X univalents are indicated by arrows. Bars = 5 μm.
In males, the late prophase I reveals a meioformula of n = 20 + X(O) (Figs 2G, 3D). There are 20 bivalents of different sizes. Some of the bivalents present two parallel homologous chromosomes. The X univalent is usually dot-shaped. A more condensed stage without chiasmata was observed near the end of prophase I (Fig. 2H). In the polar view of metaphase I (Fig. 2I), only a few bivalents present parallel-arranged homologous chromosomes.
The present work is the first cytogenetic description of the Chinese mecopteran species. In particular, we obtained first cytogenetic data for a Neopanorpa species. All the four examined species of Panorpidae possess a relatively high chromosome number, the males with an XO sex-chromosome system and achiasmatic meiosis.
Based on our study, the meioformula of males is n = 19 + X(O) in Panorpa emarginata and Panorpa dubia, n = 23 + X(O) in Panorpa sp., and n = 20 + X(O) in Neopanorpa lui. Their chromosome numbers are very similar to those of the European and American species of Panorpidae, whose meioformula is n = 22 + X(O) in Panorpa communis, Panorpa anomala and Panorpa acuta, n = 21+ X(O) in Panorpa cognata Rambur, 1842, and n = 20 + X(O) in Panorpa germanica Linnaeus, 1758 (
Compared with Panorpidae, other families of Mecoptera possess relatively low chromosome numbers. In Boreidae, males of Boreus brumalis have a meioformular of n = 11 + X1X2Y (
The precocity of X chromosome in anaphase I and the presence of dot-shaped X chromosome in late prophase I imply an XO sex-chromosome system in the four studied species. The XO sex-chromosome system also occurs in five European and American species of Panorpidae, three species of Bittacidae (
It is generally acknowledged that the formation of the XO systems is ascribed to Y chromosome degeneration from the XY system in Orthoptera and Heteroptera (
The achiasmatic meiosis in male Panorpidae has been reported several times to date (
Chiasmata are manifestations of meiotic crossovers, which not only facilitate the exchange of DNA between maternal and paternal chromosomes but also perform the important function of securing physical connections between homologous chromosomes that are essential for their co-orientation and proper disjunction at the first meiotic division (
To solve the first problem, some different modes have been proposed to facilitate the segregation of non-exchange homologous chromosomes. For example, in meiosis of male Drosophila, adhesion of homologous chromatids probably contributes to the association of homologous chromosomes (
As far as the second problem is concerned, sexual recombination is generally considered as an adaptive advantage of sexual organisms (
We thank Wen Zhong and Junxia Zhang for assistance in species identification. We also thank Na Ma and two anonymous reviewers for comments on the revision of the early draft of the manuscript. This research was supported by the National Natural Science Foundation of China (grant no. 31172125).