(C) 2013 Martin Knytl. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Knytl M, Kalous L, Ráb P (2013) Karyotype and chromosome banding of endangered crucian carp, Carassius carassius (Linnaeus, 1758) (Teleostei, Cyprinidae). Comparative Cytogenetics 7(3): 205–215. doi: 10.3897/CompCytogen.v7i3.5411
The karyotype and other chromosomal characteristics the crucian carp (Carassius carassius (Linnaeus, 1758)) were revealed by means of conventional banding protocols (C, CMA3, AgNOR). The diploid chromosome number (2n) in this species was 100. Its karyotype was composed of 10 pairs of metacentric, 18 pairs of submetacentric and 22 pairs of subtelo- to acrocentric chromosomes without any microchromosomes. C-banding identified blocks of telomeric heterochromatin on seven chromosome pairs. The NORs were situated on the p arms of the 14th pair of submetacentric chromosomes and on the p arms of the 32nd pair of subtelo-acrocentric chromosomes; AgNOR-positive signals corresponded to the CMA3-positive signals. These chromosome characteristics may suggest a paleo-allotetraploid origin of C. carassius genome.
Fish cytogenetics, paleotetraploid, heterochromatin, metaphase chromosomes
The crucian carp, Carassius carassius (Linnaeus, 1758), is a cyprinid fish that inhabits densely vegetated backwaters and oxbows of lowland rivers, shallow lakes and ponds. It is a native species to Europe with a distribution extending eastwards from the River Rhine to the River Kolyma in Siberia (
There is a number of factors that may have contributed to the disappearance of Carassius carassius, including habitat loss and degradation (
Genetic contamination seems to be a very important but hidden threat to Carassius carassius that has been recently discovered. Hybridization occurs between Carassius carassius and Carassius gibelio (
The cytogenetics of Carassius carassius is still poorly understood, since only a few studies of this speciesbased onGiemsa-stained chromosomes are known (Table 1). Interestingly, two different diploid chromosome numbers 2n = 50 and 2n = 100 were reported.
Chromosome numbers and karyotypes of Carassius carassius reported from Europe; NA = not available.
| 2n | Diploid karyotype | Locality | Source |
|---|---|---|---|
| 104 | 20m+72sm+12a | NA | |
| 100 | 20m+44sm+36a | France | |
| 100 | 52m-sm+48 st-a | Drina R., Ukrinski Lug (Bosnia) | |
| 100 | 20m+40sm+40a | the Netherlands | |
| 50 | 20m+12sm+18s-ta | lower Danube R. (Romania) | |
| 100 | 48m-sm+52st-a | Russia | |
| 100 | NA | Elbe R. System (Czech Republic) | |
| 100 | NA | Vistula R. System (Poland) | |
| 100 | 20m+36sm+44st-a | Elbe R. System (Czech Republic) | This study |
Such an unclear situation encourages us to present cytogenetic analyses of Carassius carassius with respect to ongoing hybridization processes and threats in European waters. The present study deals with chromosomal characteristics of crucian carp (Carassius carassius) from the locality Byšičky in vicinity of the Elbe River (Czech Republic). Prussian carp (Carassius gibelio) and crucian carp co-occur in this place and the a hybrid allopolyploid female with 206 chromosomes was recently discovered there (
Four females and one male werecollected during a field survey of ichthyofauna in alluvial ponds and old oxbows of the Elbe River close to the city of Lysá nad Labem (GPS: 50°10.75' N, 14°47.62' E). All five individuals were identified morphologically as common Carassius carassius (not the dwarf form)according to
All collected fish were subjected to a non-destructive procedure for chromosome preparation from fin clips developed by
CMA3, DAPI, C-banding and AgNOR images were captured with a cooled CCD camera Olympus DP30BW (equipped with a black-and-white (B&W) CCD-Chip Sony ICX285-AL) coupled to an epifluorescence microscope Olympus AX70 equipped with a set of 3 narrowband fluorescent filters. Micrographs were captured with the Olympus Acquisition Software and B&W images were processed with the software Micro Image. Altogether 200 images (metaphases), i.e. 50 images for each banding type (CMA3, DAPI, C and AgNOR) were taken and analyzed.
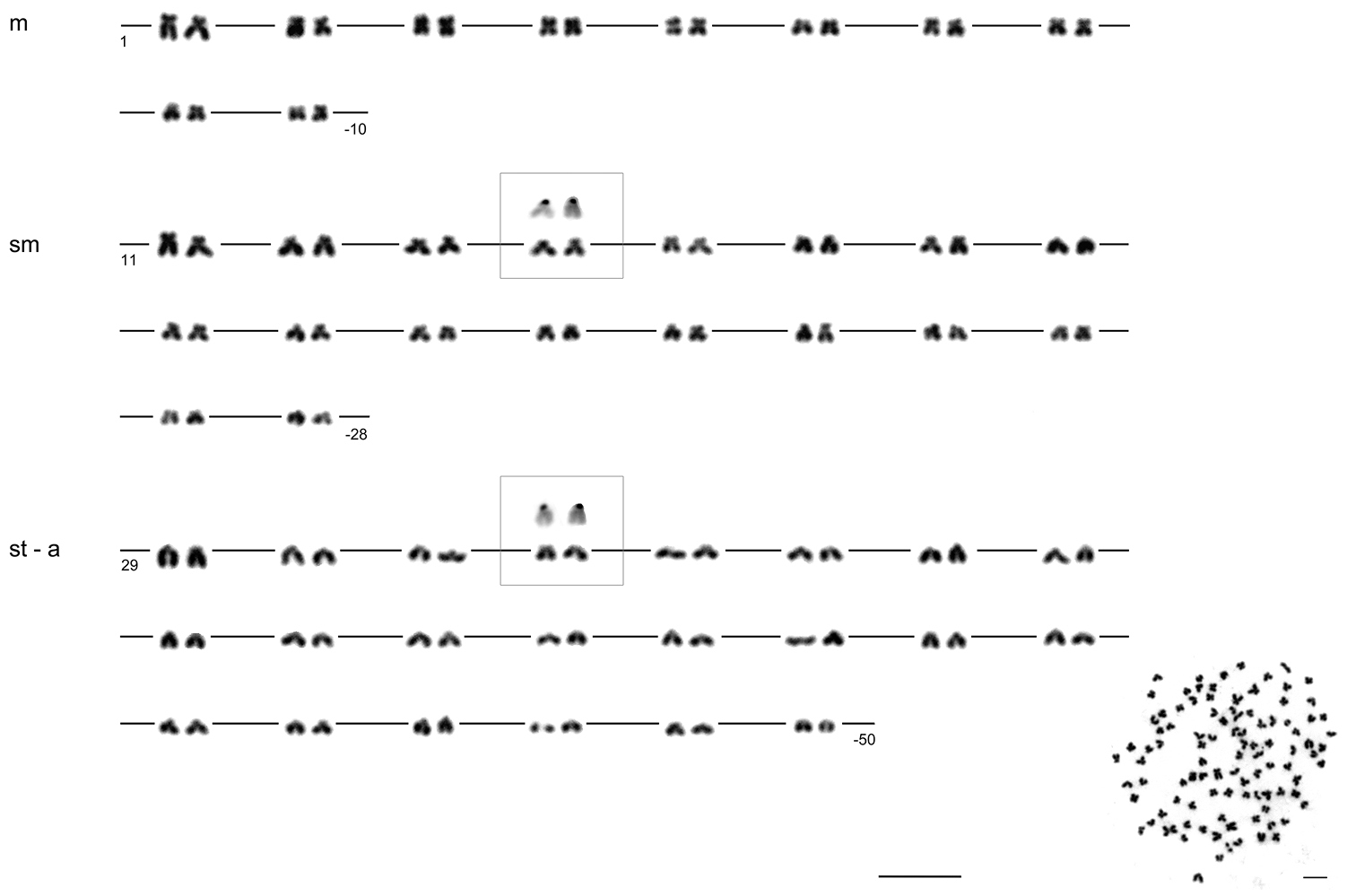
The diploid chromosome number of the examined individuals was invariably 2n = 100 (75 % investigated metaphases). The karyotype consisted of 10 pairs of metacentric (m), 18 pairs of submetacentric (sm) and 22 pairs of subtelo- (st) to acrocentric (a) chromosomes without any microchromosomes (Fig. 1).
Karyotype of Carassius carassius female arranged from Giemsa-stained chromosomes (shown as inlay); m – metacentric, s – submetacentric, st – subtelocentric, a – acrocentric chromosomes. Four CMA3-positive (color-inverted) chromosomes (14th pair of sm chromosomes and 32nd pair of st-a chromosomes) are additionally shown in the frames. Bar = 10 μm.
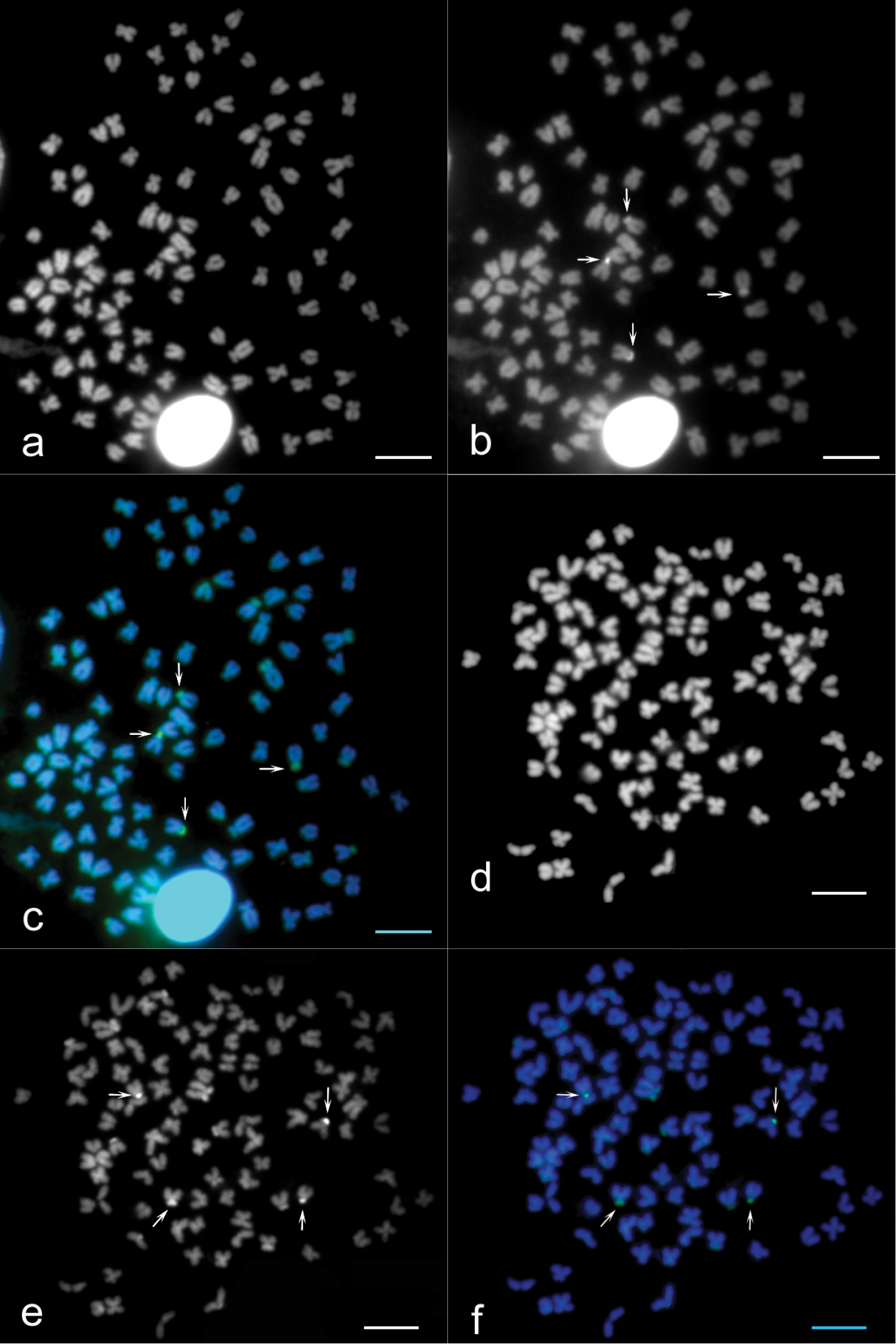
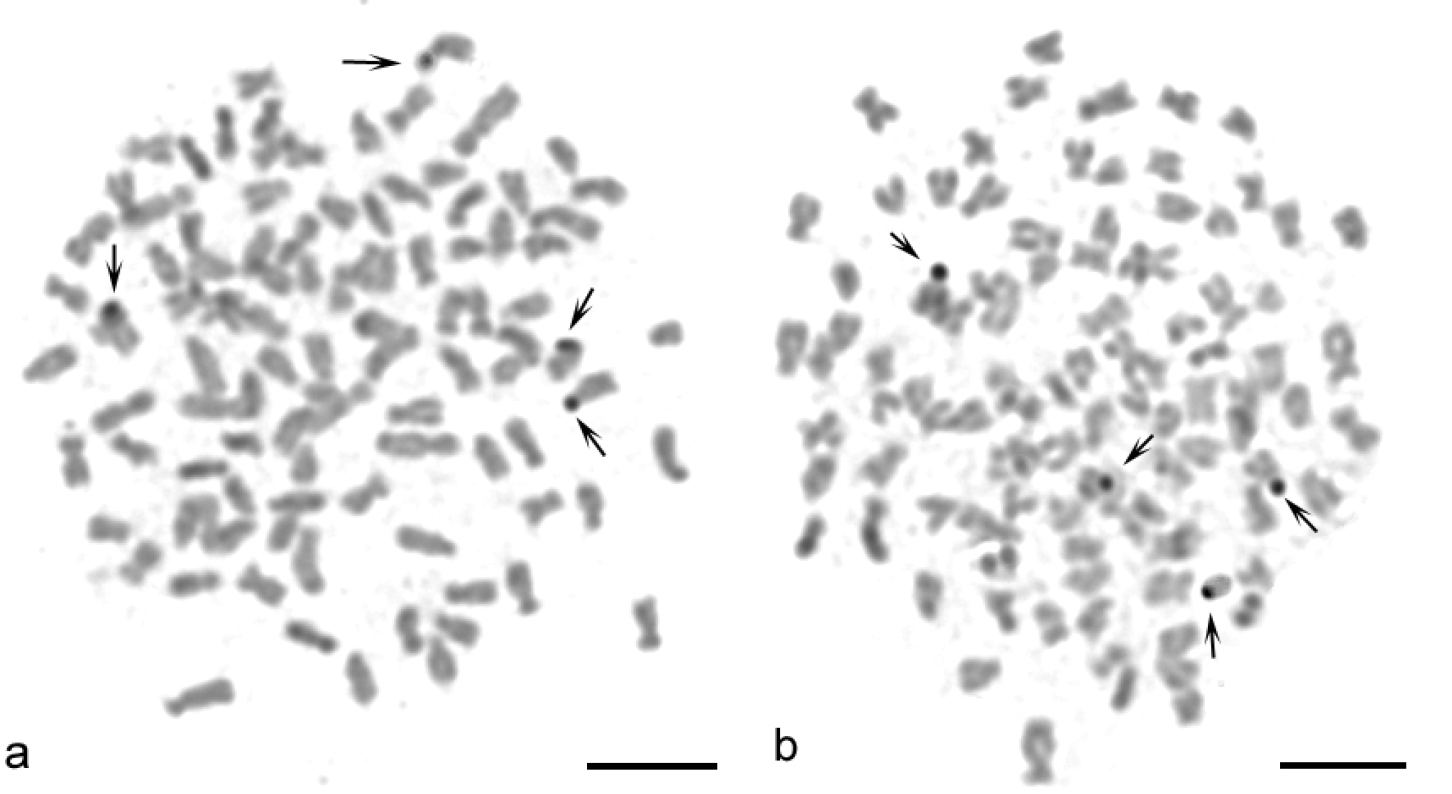
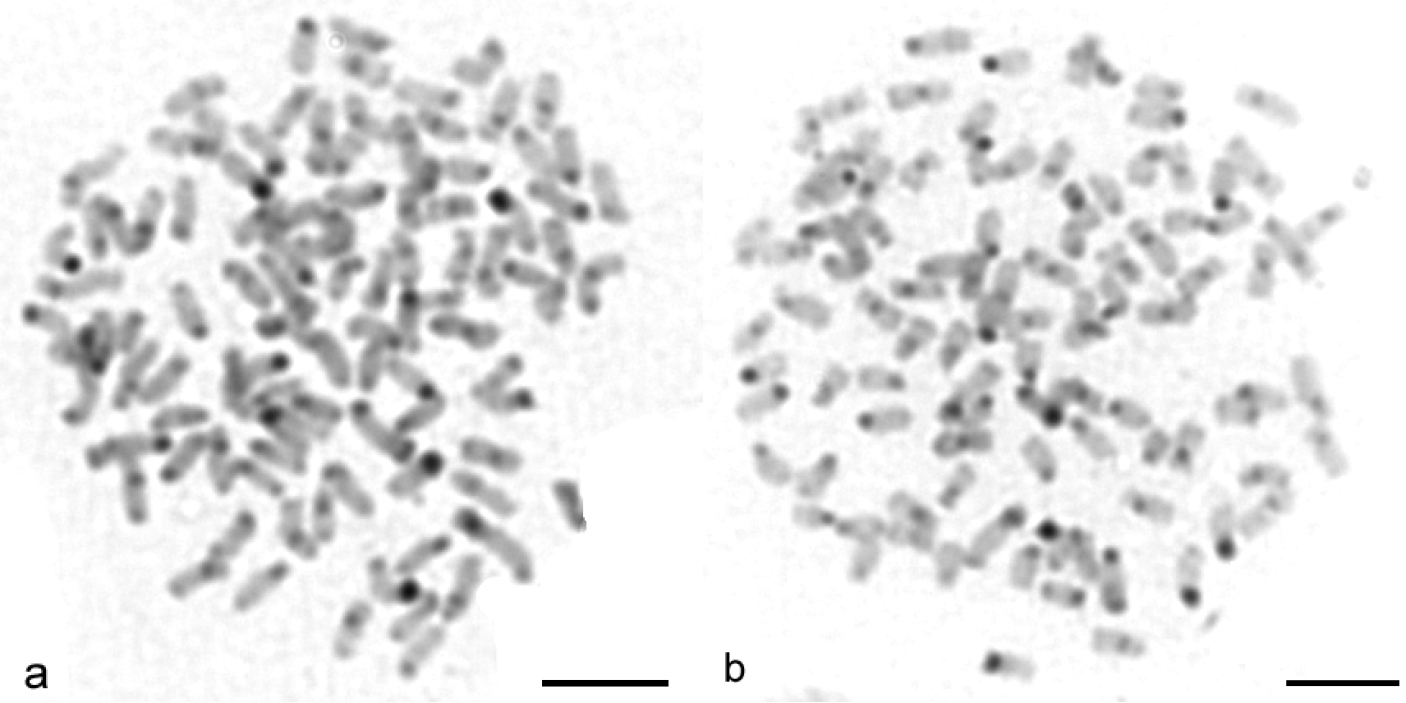
Sequential banding (DAPI + CMA3) revealed four CMA3-positive bands situated at the sites of the secondary constrictions on the p arms of the 14th pair of sm chromosomes and on the p arms of the 32nd pair of st-a chromosomes (Figs 2b, c, e, f). DAPI uniformly stained all chromosomes (Figs 2a, d). AgNOR analysis revealed four positive signals (Figs 3a, b) which corresponded to four CMA3 positive signals. C-banding detected blocks of constitutive heterochromatin at the telomeric and pericentromeric chromosome regions (Figs 4a, b). Telomeric signals were more intensive than pericentromeric ones. C-banded chromosomes were arranged in an karyotype (Fig. 5). Seven chromosome pairs had conspicuous C-banded arms.
a–f Sequential chromosome banding of Carassius carassius female chromosomes. Metaphases counterstained by DAPI show all 100 chromosomes (a, d), metaphases stained by CMA3 show 4 NORs (b, e white arrows) and the combination of these bandings show 4 identical NORs (c, f white arrows; green signals). Bar = 10 μm.
a–b AgNOR staining metaphases of Carassius carassius female (a, b black arrows) indicate 4 NOR-positive sites. Bar = 10 μm.
a–b C-banded metaphases of Carassius carassius female (a, b) show signals localized in the telocemeric and pericentromeric chromosome regions. Bar = 10 μm.
karyotype of Carassius carassius female arranged from C-banded chromosomes. Seven pairs of chromosomes show significant signals (black arrows). Bar = 10 μm.
The karyotype of all the five individuals of crucian carp from Byšičky ox-bow had the same diploid chromosome number 2n = 100. This number equalled the value reported in other previous studies (Table 1) except those by
The present study demonstrated that karyotype of individuals of Carassius carassius under study possessed 10 pairs of metacentric, 18 pairs of submetacentric and 22 pairs of subtelo- to acrocentric chromosomes, already reported by
DAPI-counterstained chromosomes did not provide any useful information since the observed signals were uniform throughout the chromosomes. Similar results were reported for Carassius gibelio by
We have performed C-banding on chromosomes of Carassius carassius for the first time. Constitutive heterochromatin blocks detected by C-banding method were located in telomeric regions of 7 pairs of chromosomes. Number of these signals can be a species-specific marker, especially in paleotetraploid forms.
Although there is no information about sex differences between Carassius carassius karyotypes, we have to point out that only one male specimen was included in this study
In respect to its status of a highly endangered fish species and unclear distribution of possible diploid and/or paleotetraploid forms as well as ongoing hybridization process with other species of this genus across its range of distribution, the present study is a moderate but important contribution to the cytogenetics and cytotaxonomy of Carassius carassius.
We thank M. Rábová and M. Pokorná for their help with the preparation of karyotypes, and we are grateful to Tomáš Daněk for the valuable information about the locality. The editor and two anonymous referees notably helped us to improve this text. The present study was supported by the S-grant MŠMT ČR and project No. P506/11/P596 of the Czech Science Foundation.