(C) 2013 Valentina G. Kuznetsova. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Kuznetsova VG, Golub NV, Aguin-Pombo D (2013) Karyotypes, B-chromosomes and meiotic abnormalities in 13 populations of Alebra albostriella and A. wahlbergi (Hemiptera, Auchenorrhyncha, Cicadellidae) from Greece. Comparative Cytogenetics 7(4): 305–325. doi: 10.3897/CompCytogen.v7i4.6411
In this work 13 populations of the leafhopper species Alebra albostriella (Fallén, 1826) (6 populations) and A. wahlbergi (Boheman, 1845) (7 populations) (Cicadellidae: Typhlocybinae) from Greece were studied cytogenetically. We examined chromosomal complements and meiosis in 41 males of A. albostriella sampled from Castanea sativa, Fagus sylvatica and Quercus cerris and in 21 males of A. wahlbergi sampled from C. sativa, Acer opalus and Ulmus sp. The species were shown to share 2n = 22 + X(0) and male meiosis of the chiasmate preductional type typical for Auchenorrhyncha. In all populations of A. albostriella and in all but two populations of A. wahlbergi B chromosomes and/or different meiotic abnormalities including the end-to-end non-homologous chromosomal associations, translocation chains, univalents, anaphasic laggards besides aberrant sperms were encountered. This study represents the first chromosomal record for the genus Alebra and one of the few population-cytogenetic studies in the Auchenorrhyncha.
Karyotype, meiosis, chromosomal associations, translocations, macrospermatids, B-chromosomes, Greek populations, Alebra, Cicadellidae, Auchenorrhyncha
The leafhopper genus Alebra Fieber, 1872 (Cicadellidae: Typhlocybinae) comprises a complex of phytophagous species with several degrees of association to deciduous trees and shrubs. This Holarctic genus is represented in Europe by six valid species and several host associated populations of unknown taxonomic status. The taxonomy of Alebra is difficult due to the very slight morphological differences in male genital structures, a significant degree of intraspecific color pattern variation and the common occurrence of two or more species on the same food plant (
Chromosomal polymorphisms in natural populations may play a significant role in speciation (
In the present work, cytogenetic analysis of Alebra albostriella (Fallén, 1826) and Alebra wahlbergi (Boheman, 1845) was performed using routine chromosome staining. A study of 13 Greek populations of these species inhabiting different deciduous trees was undertaken to reveal whether the populations of these species display any polymorphism for chromosomal complements and meiotic patterns.
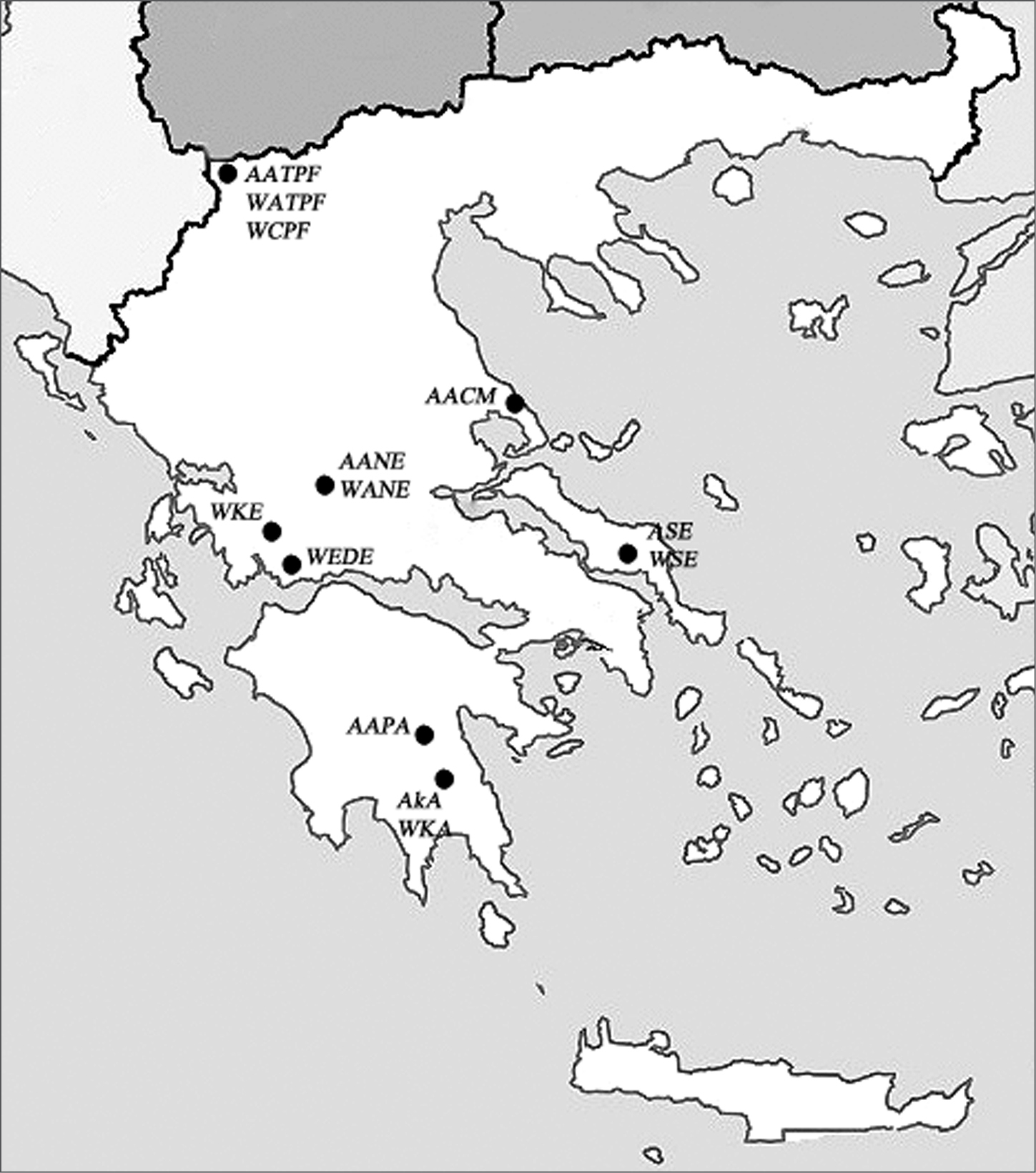
Altogether, 41 males from 6 populations of Alebra albostriella and 21 males from 7 populations of Alebra wahlbergi inhabiting 5 different species of deciduous trees in Greece have been collected from 1989 to 1992 on plant foliage with a sweeping net. The locality names, altitude, data of collection and food plants are listed in Table 1, and the places of collection are also mapped on Fig. 1. For chromosome studies, adult males were fixed in Carnoy solution (3:1 ethanol and glacial acetic acid) and stored at -10°C. Chromosomal analysis was performed using conventional squashing procedure. Testes were dissected out, stained with 2% acetic orcein and squashed in a drop of 45% acetic acid under an 18-mm square coverslip. From 1 to 14 individuals in each population were examined. Chromosome preparations were analyzed under a Leica DM 4000B microscope (Leica Microsystems Wetzlar GmbH, Germany) with a 100× objective. Images were taken with a Leica DFC 350 FX camera using Leica Application Suite 2.8.1 software with an Image Overlay module. The data obtained are presented in Tables 2–4.
Studied material.
| Species | Population code | Locality | Altitude above sea level | Food plant | Data of collection | Number of studied males |
|---|---|---|---|---|---|---|
| Alebra albostriella | ASE |
Steni-Euboea Il. | 440 m | Castanea sativa | 8–9.07.1990 | 10 |
| AKA | Kastanitsa-Arkadia | 850 m | Castanea sativa | 25.06.1990 10.08.1989 |
2 3 |
|
| AACM | Anilio-Chania-Magnisia | 990 m | Castanea sativa | 23.07.1990 | 5 | |
| AAPA | Agios Petros-Arkadia | 990 m | Castanea sativa | 15–16.7.1990 | 4 | |
| AANE | Agios Nicolaos-Eurytania | 1000 m | Castanea sativa | 01.08.1991 | 2 | |
| AATPF |
Agia Triada-Prespes-Florina | 1200 m | Fagus sylvatica | 14–21.08.1990 | 14 | |
| Quercus cerris | 20.08.1990 | 1 | ||||
| Alebra wahlbergi | WEDE | Evinos Delta-Etoloakarnania | 20 m | Ulmus sp. | 25.06.1991 | 5 |
| WSE | Steni-Euboea Il. | 440 m | Castanea sativa | 8–9.07.1990 | 2 | |
| WKE | Kerasovo-Etoloakarnania | 520 m | Acer opalus | 14.06.1992 | 4 | |
| WKA | Kastanitsa-Arkadia | 850 m | Castanea sativa | 25.06.1990 | 1 | |
| WANE | Agios Nicolaos-Eurytania | 1020 m | Castanea sativa | 01.08.1991 | 3 | |
| WCPF | Caries-Prespes-Florina | 1100 m | Acer opalus | 19.08.1990 | 1 | |
| WATPF | Agia Triada-Prespes-Florina | 1200 m | Acer opalus | 14–21.08.1990 | 5 |
*Here and elsewhere we use abbreviations to refer to different populations of a species.
** Specimens of Alebra albostriella from the AATPF locality represent two different populations one occurring on Fagus sylvatica and the other on Quercus cerris (see
Map showing the collection localities of Alebra albostriella and Alebra wahlbergi in Greece.
B-chromosomes, meiotic abnormalities and macrospermatids in Alebra albostriella.
| Populations (N=6) | Food plants | Males No (N=41) | Number of B-chromosomes | Meiotic abnormalities and macrospermatids |
|---|---|---|---|---|
| ASE | Castanea sativa | 1 | 0 | univalents |
| 2 | 0 | end-to-end non-homologous associations anaphasic laggards macrospermatids | ||
| 3 | 0 | end-to-end non-homologous associations macrospermatids | ||
| 4 | 0 | end-to-end non-homologous associations macrospermatids | ||
| 5 | 0 | anaphasic laggards macrospermatids | ||
| 6 | 0 | macrospermatids | ||
| 7 | 0 | macrospermatids | ||
| 8 | 0 | macrospermatids | ||
| 9 | 0 | - | ||
| 10 | 2 | - | ||
| AKA | Castanea sativa | 1 | 0 | univalents |
| 2 | 0 | - | ||
| 3 | 0 | - | ||
| 4 | 0 | - | ||
| 5 | 0 | - | ||
| AACM | Castanea sativa | 1 | 1 | anaphasic laggards macrospermatids |
| 2 | 0 | macrospermatids | ||
| 3 | 0 | macrospermatids | ||
| 4 | 0 | macrospermatids | ||
| 5 | 0 | - | ||
| AAPA | Castanea sativa | 1 | 0 | macrospermatids |
| 2 | 0 | - | ||
| 3 | 0 | - | ||
| 4 | 0 | - | ||
| AANE | Castanea sativa | 1 | 1 | end-to-end non-homologous associations univalents anaphasic laggards |
| 2 | 0 | end-to-end non-homologous associations anaphasic laggards macrospermatids | ||
| AATPF | Fagus sylvatica | 1 | 1 | macrospermatids |
| 2 | 0 | univalents macrospermatids | ||
| 3 | 0 | end-to-end non-homologous associations | ||
| 4 | 0 | macrospermatids | ||
| 5 | 0 | macrospermatids | ||
| 6 | 0 | macrospermatids | ||
| 7 | 0 | macrospermatids | ||
| 8 | 0 | macrospermatids | ||
| 9 | 0 | macrospermatids | ||
| 10 | 0 | macrospermatids | ||
| 11 | 0 | macrospermatids | ||
| 12 | 0 | macrospermatids | ||
| 13 | 0 | macrospermatids | ||
| 14 | 0 | - | ||
| Quercus cerris | 15 | 0 | anaphasic laggards |
B-chromosomes, meiotic abnormalities and macrospermatids in Alebra wahlbergi.
| Populations (N=7) | Food plants | Males No (N=21) | Number of B-chromosomes | Meiotic abnormalities and macrospermatids |
|---|---|---|---|---|
| WEDE | Ulmus sp. | 1 | 2 | univalents |
| 2 | 0 | end-to-end non-homologous associations macrospermatids | ||
| 3 | 0 | macrospermatids | ||
| 4 | 0 | - | ||
| 5 | 0 | - | ||
| WSE | Castanea sativa | 1 | 0 | univalents |
| 2 | 0 | end-to-end non-homologous associations | ||
| WKE | Acer opalus | 1 | 2 | macrospermatids |
| 2 | 0 | univalents | ||
| 3 | 1 | - | ||
| 4 | 0 | - | ||
| WKA | Castanea sativa | 1 | 0 | - |
| WANE | Castanea sativa | 1 | 0 | end-to-end non-homologous associations multiple translocation chains |
| 2 | 0 | end-to-end non-homologous associations | ||
| 3 | 0 | univalents | ||
| WCPF | Acer opalus | 1 | 0 | - |
| WATPF | Acer opalus | 1 | 0 | macrospermatids |
| 2 | 0 | - | ||
| 3 | 0 | - | ||
| 4 | 0 | - | ||
| 5 | 0 | - |
Frequency of B chromosomes in Alebra albostriella and Alebra wahlbergi.
| Male N=7 | Number of B chromosomes per cell | Total number of MI studied | Number of MI with B chromosomes | Frequency of B chromosomes per individual, % |
|---|---|---|---|---|
| Alebra albostriella | ||||
| 1-AACM | 1 | 460 | 3 | 0, 65 |
| 1-AANE | 1 | 370 | 4 | 1, 08 |
| 10-ASE | 2 | 98 | 82 | 83, 7 |
| 1-AATPF | 1 | 112 | 2 | 1, 8 |
| Alebra wahlbergi | ||||
| 1-WEDE | 2 | 28 | 17 | 60, 7 |
| 1-WKE | 2 | 107 | 84 | 78, 5 |
| 3-WKE | 1 | 180 | 2 | 1, 1 |
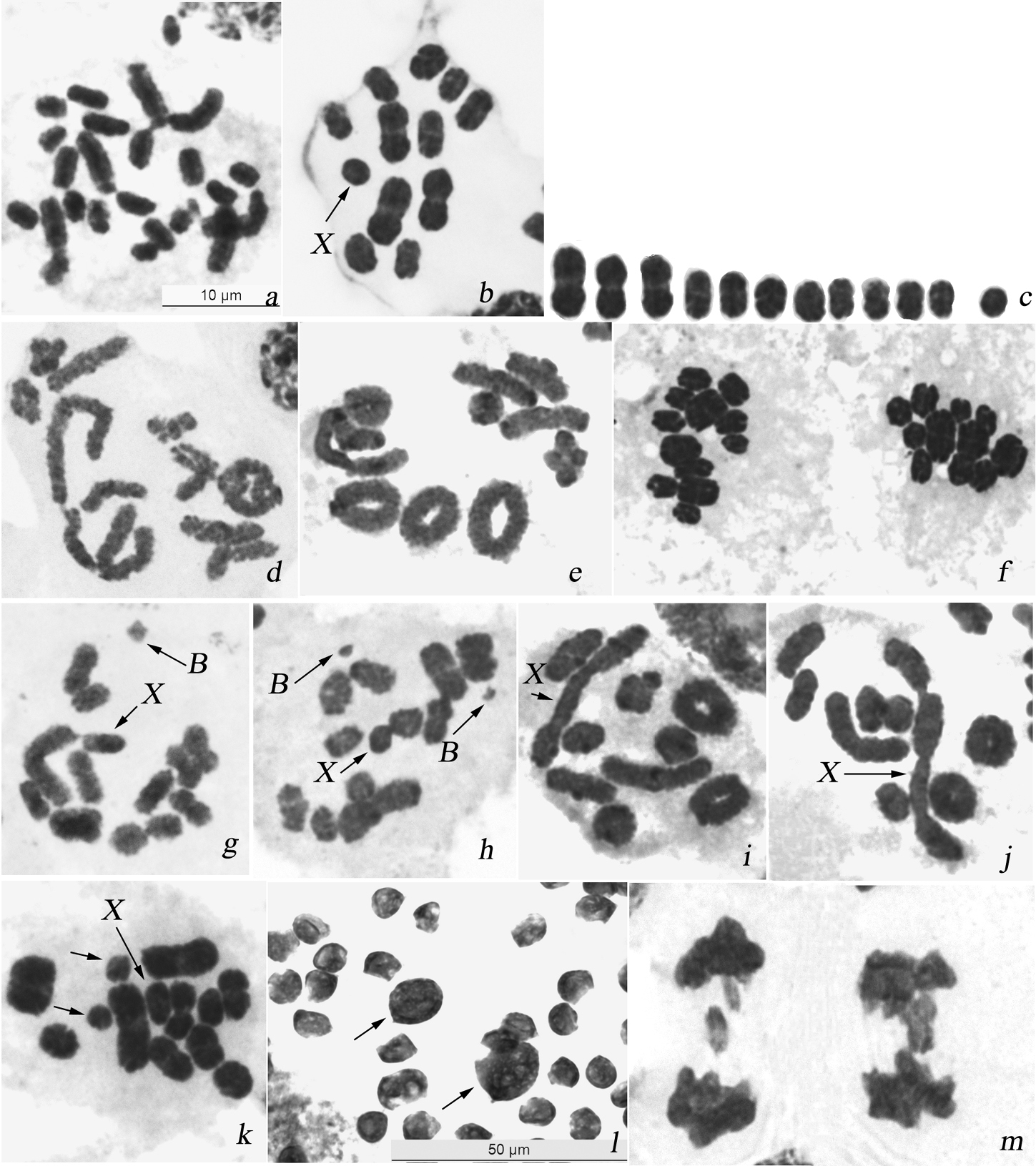
In males, the majority of cells showed 23 chromosomes at mitotic metaphases (Fig. 2a) and 12 units at meiotic metaphases I (MI) (Fig. 2b, c). The karyotype is asymmetric with two size groups of chromosomes. In mitosis six larger chromosomes and other chromosomes constituting a decreasing series in size were present. Chromosomes had no primary constrictions, i.e. centromeres and the sex chromosome could not be identified. At MI, 11 autosomal bivalents, including three larger, and a univalent X-chromosome were encountered (n=11 + X). Male karyotype formula of the species is thus as follows: 2n=22 + X(0). The univalent X-chromosome was similar in size to one of the larger half-bivalents within the group of smaller chromosomes and its location at MI was random. Bivalents mainly had a single terminal/subterminal or, rarer, interstitial chiasma, however in few nuclei up to four rings were present indicative of two terminal/subterminal chiasmata being formed in the larger bivalents (Fig. 2d, e). At anaphase I (AI), all the autosomes segregated to opposite poles and the X moved to one pole without dividing. The reductional division resulted thus in two daughter metaphase II (MII) cells with 11A+X and 11A, respectively (Fig. 2f).
Karyotype and male meiosis in Alebra albostriella: a Mitotic metaphase showing 23 chromosomes b MI showing 11 bivalents and univalent X c karyogram prepared from MI (b) d diakinesis showing bivalents with one terminal/subterminal chiasma and a bivalent with two subterminal chiasmata e diakinesis showing bivalents with one terminal/subterminal chiasma, a bivalent with interstitial chiasma and 4 ring bivalents each with two terminal/subterminal chiasmata f two daughter AI with n=11 and n=12, respectively g diakinesis showing one B chromosome h diakinesis showing two B chromosomes i diakinesis with end-to-end association of two bivalents and X j diakinesis with end-to-endassociation ofthree bivalents and X k MI with one medium-sized bivalent as univalents (arrowed) l macrospermatids of different size (arrowed) among normal spermatids m AI with lagging chromosomes. Bar = 50 µm in l and 10 µm in other figures.
In 4 out of 20 males analyzed in the populations ASE, AACM, AANE (sampled from Castanea sativa) and AATPF (sampled from Fagus sylvatica) one or two small B-chromosomes (additional to the standard complement) were found (Table 2). In the polymorphic populations, three males (1-AACM, 1-AANE and 1-AATPF) showed a single B-chromosome with the frequency of about 1% per specimen while male 10-ASE had a pair of B-chromosomes in about 80% of MI (Table 4). B-chromosomes were different in size in different males while always appreciably smaller than the X-univalent and negatively heteropycnotic at late prophase and MI (Fig. 2g, h). At MI, B-chromosome(s) showed random distribution relative to autosomal bivalents and X-chromosome. In the case of two B-chromosomes, they did not show any connection to each other (Fig. 2h).
Different kinds of meiotic abnormalities were encountered in 12 males (29% of the total number of males) sampled from all the 6 populations (Table 2). In males 2, 3 and 4 of ASE (from Castanea sativa), in both studied males of AANE (from Castanea sativa), and in male 3 of AATPF (from Fagus sylvatica) two to four bivalents were occasionally associated by ends. The univalent X-chromosome was very often involved in these associations. Non-homologous telomeres did not touch intimately each other but unstained gaps were seen between the bivalents (Fig. 2i, j). In some males (Table 2), one or two middle-sized bivalents were seen as univalents in some cells at diakinesis and MI (Fig. 2k). In addition, populations ASE, AACM, AANE and AAPA (from Castanea sativa) and AATPF (from Fagus sylvatica) the majority of studied males (61%; N=25) showed macrospermatids coexisting with normal spermatids within a cyst. Macrospermatids were different in size being either approximately twice larger or much larger than the normal spermatids (Fig. 2l). Some males with aberrant spermatids displayed also one or other type of meiotic abnormalities, including lagging chromosomes at anaphases (Fig. 2m) (Table 2).
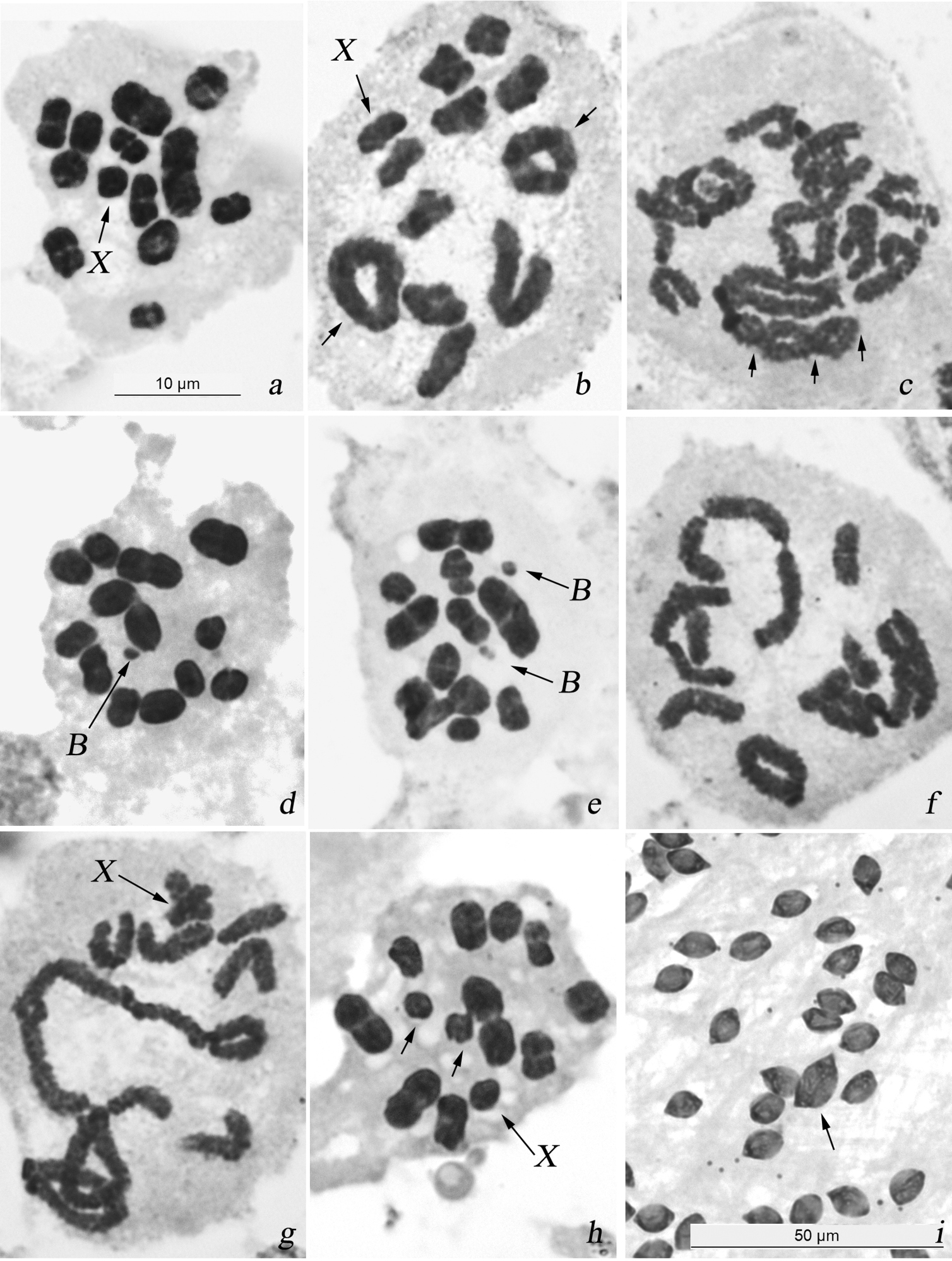
In sampled males, 11 autosomal bivalents and the univalent X-chromosome were present in cells at diakinesis and MI (Fig. 3a, b), male karyotype formula of this species being thus 2n = 22 + X(0). Much as in Alebra albostriella, this karyotype was asymmetric with three larger bivalents and 8 smaller bivalents, the X-chromosome being similar in size to one of the larger half-bivalents in this second group. The chromosomes had no centromeres. The univalent X-chromosome was located randomly at diakinesis and MI. The bivalents mainly had a single terminal/subterminal or rarely interstitial chiasma, however, two chiasmata (rings on Fig. 3b) and occasionally three chiasmata (arrowed on Fig. 3c) could be formed in larger bivalents. In few cells at diakinesis and metaphase I four bivalents or (in male 1-WNE) even six bivalents with two chiasmata each were observed (not shown). Both autosomes and X-chromosome separated reductionally during the first division and divided equationally during the second division of meiosis.
Karyotype and male meiosis in Alebra wahlbergi: a MI showing 11 bivalents and univalent X b diakinesis showing bivalents with one terminal/subterminal chiasma, a bivalent with interstitial chiasma and 2 ring bivalents each with two terminal/subterminal chiasmata c diplotene/diakinesis showing a bivalent with three (at least) chiasmata (arrowed) d MI with one B chromosome e MI with two B chromosomes f diakinesis with end-to-end association of three bivalents g diplotene/diakinesis showing translocation chain involving 4 bivalents h MI with one medium-sized bivalent as univalents (arrowed) i macrospermatid (arrowed) among normal spermatids. Bar = 50 µm in i and 10 µm in other figures.
In 3 out of 9 males analysed in the populations WEDE (sampled from Ulmus sp.) and WKE (sampled from Acer opalus) one or two B-chromosomes were encountered (Table 3). The polymorphic male 3-WKE showed a single B-chromosome in about 1% of MI (Fig. 3d, Table 4). Males 1-WEDE and 1-WKE showed each a pair of Bs at MI with frequencies of approximately 60% and 80%, respectively (Fig. 3e, Table 4). In every case B-chromosomes were very small, negatively heteropycnotic and distributed randomly with reference to each other, to the bivalents and to the X-chromosome.
Meiotic abnormalities were encountered in 11 males (52% of the total number of males) sampled from 5 out of 7 studied populations. Populations WKA and WCPF showed no meiotic disturbances however in our study they were represented by only one male each (Table 3). In males 2-WEDE, 2-WSE, 1- and 2-WANE the bivalents occasionally formed associations involving two or three bivalents connected by telomere ends. Non-homologous telomeres did not touch intimately each other but unstained gaps were seen between the bivalents (Fig. 3f). In addition, male 1-WANE had nuclei at diakinesis with X-chromosome, 7 bivalents and a translocation chain of four bivalents united by chiasmata (Figs 3g). The chromosomal complement of these cells was in fact n = 7AA + 1(4AA) + X. Unfortunately, because of poor spreading of chromosomes in the slide no statistical analysis of the occurrence of translocation chains in this male was possible. Further still, we failed to detect the number of bivalents involved into certain translocation chains. In males 1-WEDE, 1-WSE, 2-WKE and 3-WANE, one of the middle-sized bivalents was present as univalents at MI (Fig. 3h). In populations WEDE, WKE and WATPF, 4 out of 14 males showed macrospermatids which were of approximately twice the normal size and coexisted with normal spermatids within a cyst (Fig. 3i). Among males with macrospermatids, only that 2-WEDE displayed meiotic disturbances namely the end-to-end non-homologous associations of bivalents (Table 3).
Alebra albostriella and Alebra wahlbergi were found to have the same karyotype of 2n = 22 + X(0) encountered without variation in 30, 6% of studied males (N=19) and with some variation due to polymorphism in the rest of males (N=43) . The species share likewise a similar gross morphology of karyotypes. Both karyotypes are asymmetric in terms of the heterogeneity of chromosome size: one size group includes three pairs of larger chromosomes and the other group includes 8 pairs of smaller chromosomes. Within every group, chromosomes represent continuous gradation in size and therefore can not been reliably distinguished by conventional cytogenetic approaches. The X-chromosome is close by size to one of the larger chromosomes within the smaller-sized group. Chromosomes are holokinetic as in other Hemiptera; that is, the centromeric activity is dispersed along the length of each chromosome rather than concentrated at one point (
In spite of holokinetic nature of chromosomes, there are only a few Cicadellidae genera in which chromosome number has been sufficiently liable to change in the course of speciation whereas most genera have a stable number of chromosomes (
Cytological analysis of male meiosis in Alebra albostriella and Alebra wahlbergi revealed that it was of the typical auchenorrhynchan type where all the chromosomes undergo segregation at anaphase I and chromatids separate at anaphase II (
In the majority of auchenorrhynchans, at least in all hitherto studied planthopper species (Fulgoroidea), the univalent X-chromosome shows a clear tendency to be arranged at the periphery of MI plate presumably forming its own meiotic spindle (
Polymorphism for chromosomal rearrangements, B-chromosomes and meiotic abnormalities are not rare in nature and has been recorded for numerous species of plants and animals, including insects (
B-chromosomes are accessory genomic elements that are known to occur approximately in 15% of living species (
In studiedpopulations, the frequency of B chromosomes was rather low. Thus, in Alebra wahlbergi they were present in 14% and in Alebra albostriella in only 10% of specimens studied. The frequency of individuals with 1B and 2Bs differed between the species. Thus, in four Alebra albostriella populations with B-chromosomes, 9, 6% of males carried 1B and 3, 2 % carried 2B, whereas in two Alebra wahlbergi populations with Bs, 11% of males had 1B and 22% had 2B. The data concerning the frequency of B chromosomes in natural populations of these species are too scarce to draw any firm conclusions. Noteworthy that the frequency of cells with B-chromosomes in 2B-males was markedly higher compared with that in 1B-carriers: 60, 7%-83, 7% against 0, 65%-1, 8% (Table 4). This observation suggests the existence of an accumulation mechanism responsible for maintaining the 2Bs in studied populations. Interesting, no males with more than two Bs were found in studied specimens. One can suppose that in Alebra populations, 1 or 2 B-chromosomes are tolerable to B-carriers and that natural selection operates by eliminating individuals with more than two Bs. In contrast, in the aforesaid planthopper species Javesella pellucida, males with up to four B-chromosomes were found (
The question of the adaptive significance of B-chromosomes in natural populations has been argued over for decades (
As noted above, information on chromosome rearrangements and meiotic disturbances in auchenorrhynchan species is very scarce. A number of meiotic abnormalities including agmatoploidy (a result of fission of holokinetic chromosomes), aneuploidy, loose pairings of sex chromosomes and shrinkage of cytoplasm (changes in cytoplasmic volume) were described in three biotypes of the brown planthopper Nilaparvata lugens (Stål, 1854) from the family Delphacidae (
In males originating from different populations of Alebra albostriella and Alebra wahlbergi, end-to-end associations between bivalents were found at different stages of meiosis. The chains, involving up to four bivalents and occasionally (in Alebra albostriella) also the X-chromosome, were formed during prophase and were still intact at MI. In these cases, the persistent association was certainty non-chiasmate. Non-homologous telomeres did not touch intimately each other but unstained gaps were present between bivalents. Since in both species chromosomes display distal heterochromatic blocks (our unpublished data), one can suggest that the formation of artificial bivalent chains (pseudomultiples) is caused by heterochromatin adhesion due to which non-homologous chromosomes easily attract to one another.
Terminal associations of non-homologous bivalents without chiasma formation have been described in many plants and animals (
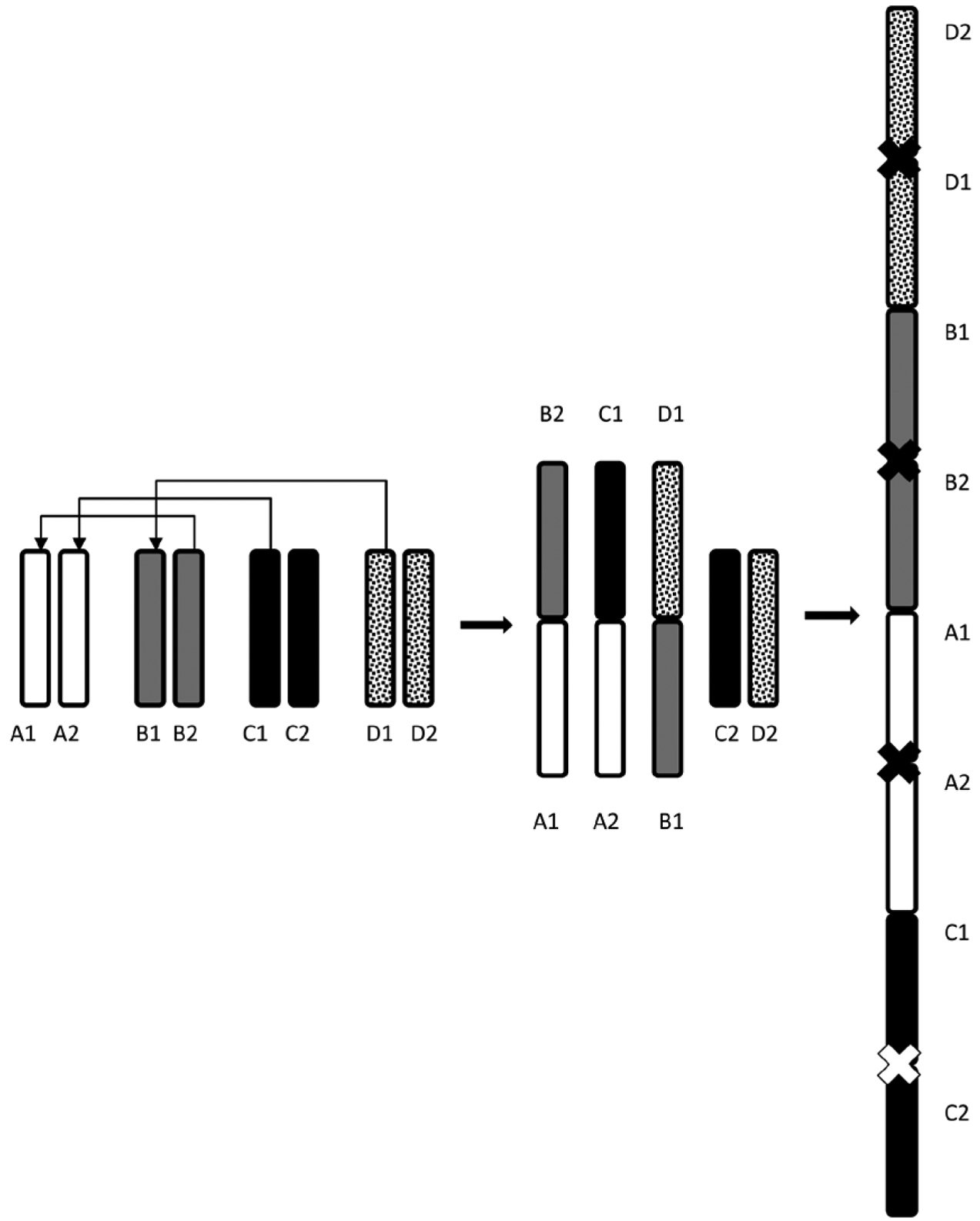
In male 1-WANE sampled from Castanea sativa, part of nuclei had chains of several bivalents, non-homologous chromosomes showing the apparent intimate contacts by terminal chiasmata (Fig. 3g). In this male, the occurrence of heterozygotes for translocations could account for the multivalent chains formation. Since this chromosome rearrangement was observed only in some of meiotic cells, it must have happened after germinal cell development. Unfortunately, using only classical cytogenetic methods it was possible neither to affirm which chromosomes formed the chains nor to identify the orientation of separate chromosomes within a chain. Schematic representation of the possible formation of the chain-of-four caused by multiple translocations in meiotic cells is presented on Fig. 4.
Schematic representation of the possible formation of a multiple translocation chain of four bivalents in meiosis of 1-WANE male. A1A2, B1B2, C1C2 and D1D2 are autosomal bivalents consecutively involved in translocation. Chiasmata in a translocation chain are shown by crosses.
In natural populations, chromosomal rearrangements arise as heterozygotes but their probability to establish is low (
In separate males of Alebra albostriella and Alebra wahlbergi, univalents of one-two chromosome pairs were observed at MI. The univalency involved either one of the larger or one of the smaller pairs of autosomes or occasionally both of these pairs. Although synaptic abnormalities can be responsible for the induction of abnormal chromosome segregation, no abnormal spermatids were observed in males showing univalents at metaphase I cells. This observation suggests a regular segregation of univalents in meiosis as it has been demonstrated by
Macrospermatids were encountered in males of both species. Aberrant spermatids occurred in small proportion in part of spermatocysts and were twice and sometimes several times as much as normal spermatids within the same cyst. In Alebra albostriella, abnormal spermatids were more abundant being found in four populations and in about 37% of the specimens studied. Chromosomal abnormalities that affect gametogenesis are known to be one of the principle causal factors in nonbalanced gametes appearance (
It is not known at this stage what are the primary causes of abnormal chromosome behavior in males of Alebra albostriella and Alebra wahlbergi from Greek populations i.e. whether these causes are male-specific meiotic mutants, some environmental mutagens or the result of hybridization events between coexisting species on the same tree. Also it is not known whether these meiotic abnormalities may play a role in the karyotype evolution and speciation of the genus Alebra. This genus seems to be prone to chromosomal rearrangements that makes it an interesting group for further studies. From the cytological viewpoint, a greater number of species and samples as well as more detailed analysis employing special techniques like chromosome bandings and fluorescent in situ hybridization may help in determining the actual variety and frequency of chromosomal abnormalities and their contribution into the karyotype differentiation in the genus Alebra.
This study was supported (for VK and NG) by the RFBR (grant 11-04-00734) and programs of the Presidium of RAS “Gene Pools and Genetic Diversity” and “Origin of the Biosphere and Evolution of Geo-biological Systems”, and (for DA and VK) by the FCT research project “Origin of multiple parthenoforms of Empoasca leafhoppers in Madeira Island” (PTDC/BIA-BEC/103411/2008). We thank S. Nokkala for making a valuable contribution to the interactive discussion of the meiotic images and Mss N. Khabazova for technical assistance. We wish to thank Costas Krimbas for laboratory facilities and Sakis Drosopoulos for suggesting chromosome studies on Alebra. We are also grateful to Christos Gantzias for his help in fieldwork and to Planton Andritsakis and Lili Andritsakis for providing sampling facilities.