(C) 2013 Fatemeh Afshari. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Afshari F, Ebrahimi M, Akbari M, Farajpour M (2013) Cytological investigations and new chromosome number reports in yarrow (Achillea millefolium Linnaeus, 1753) accessions from Iran. Comparative Cytogenetics 7(4): 271–277. doi: 10.3897/CompCytogen.v7i4.6075
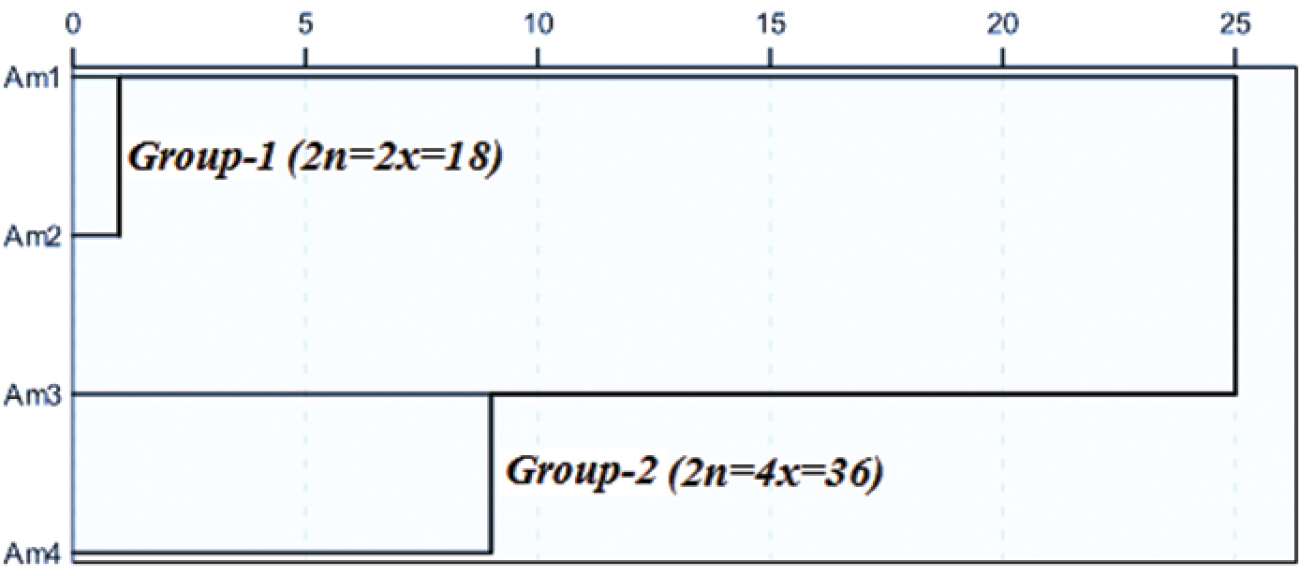
In this study, a new chromosome number for Iranian yarrow (Achillea millefolium L.) accessions was reported. Cytological analyses on four Achillea millefolium accessions, indicated that two accessions were diploids (2n=2x=18) and two tetraploids (2n=4x=36). Cluster analysis based on chromosomal characteristics and karyotype asymmetry, categorized the four accessions separated into two groups. In terms of the Stebbins’ system, the karyotype of diploid accessions grouped in 2A class. The average value of the total form percentage (TF%) in the group one (diploid accessions) and two (tetraploid accessions) were 40.85 and 41.15, respectively. The group one had the highest mean value for the symmetry index (S%=57.5). Consequently, it can be inferred that diploids belonging to the group one are the earlier evolutionary forms.
Achillea millefolium, karyotype, chromosome number, ploidy level
Achillea is one of the most recent genera of the Asteraceae family which exists throughout the world (
Achillea millefolium has a high genetic and phenotypic variation in Iran (
In most of the chemotypes in Achillea sp, camphor, borneol (
Achillea millefolium has been cytologically analyzed extensively in different regions of the world (
The aerial parts of the four Achillea millefolium accessions were collected from three provinces in north, west and south of Iran (Table 1). Voucher samples were deposited at the herbarium of Research Institute of Forests and Rangeland (RIFR) of Tehran, Iran. Seeds were germinated on moist filter paper in Petri dishes. Actively growing root tips, 1 to 2 cm length were cut from the germinating seeds and pretreated with 8-hydroxyquinoline (0.002M) for 2 to 4 hours and fixed in Farmer (1:3, glacial acetic acid : ethanol 95%) for 24 hours at 4° C. Thereafter, the root tips were hydrolyzed in 1 N NaOH at 60° C for 5-10 minutes, stained for 45 minutes in esterase stain at 30° C, and squashed in 45% glacial acetic acid. Finally, the chromosome images were obtained with photomicroscope.
Accessions of Achillea millefolium studied.
| Accessions no. | Location | Latitude, Longitude | Elevation (m.a.s.l.) |
|---|---|---|---|
| Am1 | Iran, Ardabil, Ardabil | 38°15'N, 48°17'E | 1332 |
| Am2 | Iran, Ardabil, Meshkin-Shahr | 37°58'N, 48°58'E | 1723 |
| Am3† | Iran, Ilam, Ilam | 34°27'N, 46°25'E | 1387 |
| Am4 | Iran, Fars, Estahban | 29°12'N, 53°04'E | 1767 |
†some of the characteristics of this accession were reported by
Karyotypec characteristics such as differences of range relative length (DRL), mean chromosome length (MCL), and mean arm ratio (MAR) were calculated using MICROMEASURE (Version 3.3) Software (
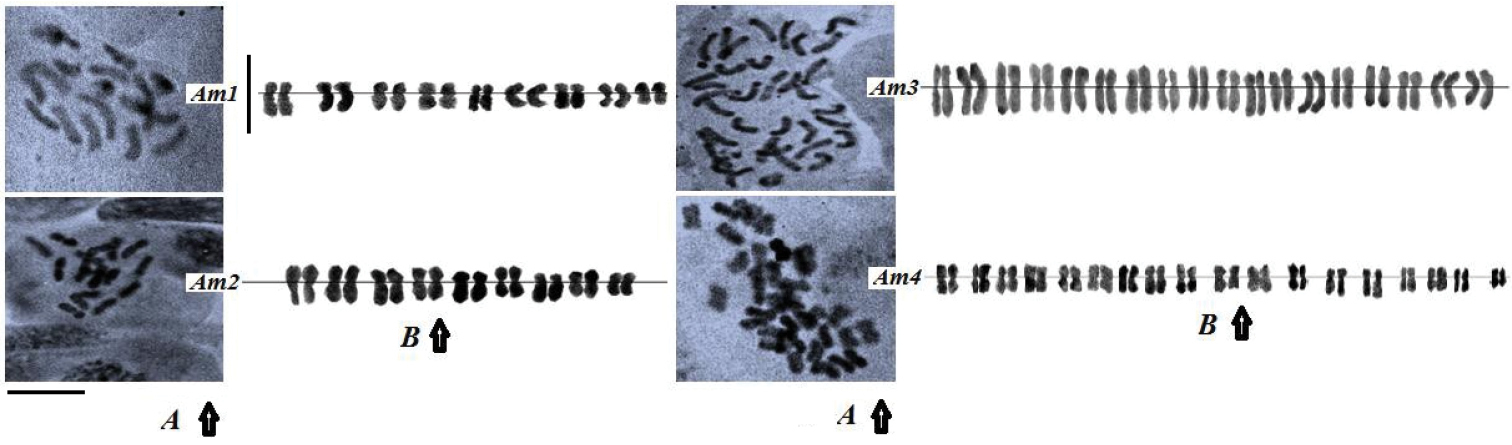
Am1 and Am2 accessions were diploids (2n= 2x= 18) whereas the two other accessions (Am3 and Am4) showed tetraploid (2n= 4x= 36) level (Figure 1). According to previous studies, Farsi et al. (2009) and
A–B Mitotic metaphases (A) and karyograms (B) of four Achillea millefolium accessions (Am1-Am4). Bars = 5μm.
Karyotypic analysis indicated asymmetrical pattern in the four accessions of Achillea millefolium (Table 2). Mitotic metaphases and karyograms of the four accessions are shown in Figure 1. The highest TCL value was found in Am3 (60.9 µm) and the lowest was found in Am2 (24.5 µm) (Table 3). The lowest and the highest DRL values were found in Am3 and Am2 accessions, respectively (Table 3). High DRL value leads to more changes in the construction of chromosomes. DRL values in the two diploid accessions were higher than the tetraploid ones; it can be a relationship between ploidy level and DRL value. The tetraploid accessions had the most symmetric karyotypes.
Karyotype features of four Achillea millefolium accessions.
| Accession | 2n | Ploidy level | TF% | S% | Karyotype formulae |
|---|---|---|---|---|---|
| Am1 | 18 | 2x | 40.9 | 64 | 1M+8m |
| Am2 | 18 | 2x | 40.8 | 51 | 1M+6m+2sm |
| Am3 | 36 | 4x | 40.5 | 63 | 16M+2sm |
| Am4 | 36 | 4x | 41.8 | 37 | 17M+1sm |
| Mean ofgroup1 | - | - | 40.85 | 57.5 | - |
| Mean ofgroup2 | - | - | 41.15 | 50 | - |
‡ TF%=total form percentage (sum of short arm lengths of individual/total haploid length of the complement chromosomes), S% -symmetry index (shorter chromosome length / longer chromosome length), karyotype formula (m, median region; sm, submedian region; M, median point).
Total chromosome length (TCL), mean chromosome length (MCL), mean arm ratio (MAR), difference of range relative length (DRL), chromosome length range (CLR), Symmetry Classes of Stebbins (SC) of four Achillea millefolium accessions.
| Accession | TCL( µm) | MCL (µm) (±SD) | MAR( µm) | DRL (µm) | CLR (µm) | SC |
|---|---|---|---|---|---|---|
| Am1 | 26.4 | 2.93(±0.13) | 0.71 | 4.92 | 2.4-3.7 | 2A |
| Am2 | 24.5 | 2.72(±0.17) | 0.69 | 6.9 | 1.8-3.5 | 2A |
| Am3 | 60.8 | 3.37(±0.09) | 0.68 | 2.2 | 2.6-4.1 | 1A |
| Am4 | 41 | 2.27(±0.11) | 0.73 | 4.49 | 1.1-2.9 | 1B |
Other parameters that indicate karyotype asymmetry are total form percentage (TF %;
Cluster analysis was done based on karyotypic characteristics (TCL, MCL, MAR and DRL) and karyotype asymmetry (TF% and S%) (Figure 2) and agrees with
Dendrogram of four Achillea millefolium accessions based on the karyotype characteristics and asymmetry.