(C) 2013 David Sadílek. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Sadílek D, Šťáhlavský F, Vilímová J, Zima J (2013) Extensive fragmentation of the X chromosome in the bed bug Cimex lectularius Linnaeus, 1758 (Heteroptera, Cimicidae): a survey across Europe. Comparative Cytogenetics 7(4): 253–269. doi: 10.3897/CompCytogen.v7i4.6012
Variation in the number of chromosomes was revealed in 61 samples of Cimex lectularius Linnaeus, 1758 from the Czech Republic and other European countries, hosted on Myotis Kaup, 1829 (4) and Homo sapiens Linnaeus, 1758 (57). The karyotype of all the specimens of Cimex lectularius analysed contained 26 autosomes and a varying number of the sex chromosomes. The number of sex chromosomes showed extensive variation, and up to 20 fragments were recorded. Altogether, 12 distinct karyotypes were distinguished. The male karyotypes consisted of 29, 30, 31, 32, 33, 34, 35, 36, 37, 40, 42 and 47 chromosomes. The females usually exhibited the number of chromosomes which was complementary to the number established in the males from the same sample. However, 11 polymorphic samples were revealed in which the karyotypes of females and males were not complementary each other. The complement with 2n = 26+X1X2Y was found in 44% of the specimens and 57, 4% samples of bed bugs studied. The karyotypes with higher chromosome numbers as well as individuals with chromosomal mosaics were usually found within the samples exhibiting particularly extensive variation between individuals, and such complements were not found within samples contaning a few or single specimen. The occurrence of chromosomal mosaics with the karyotype constitution varying between cells of single individual was observed in five specimens (4.3%) from five samples. We assume that polymorphism caused by fragmentation of the X chromosome may result in meiotic problems and non-disjunction can produce unbalanced gametes and result in lowered fitness of individuals carrying higher numbers of the X chromosome fragments. This effect should be apparently enhanced with the increasing number of the fragments and this may be the reason for the observed distribution pattern of individual karyotypes in the studied samples and the rarity of individuals with extremely high chromosome numbers. The assumed lowering of the fitness of individuals carrying higher numbers of the X chromosome fragments could affect population dynamics of variable populations.
Cimex lectularius, Cimex pipistrelli, cytogenetics, chromosome number variation, X chromosome
The genus Cimex Linnaeus, 1758 is the best known taxon of the family Cimicidae (Heteroptera) which contains up to 110 described species of haematophagous ectoparasites exploiting mostly bats and birds as hosts (
Karyotypic variation within the family Cimicidae and the genus Cimex is believed to be frequently related to the sex chromosomes. The XY sex determination system was proposed as ancestral in 53 species of cimicids that have been studied cytogenetically so far, and the diploid number in male complements varies from 2n=10 to 47, with the modal number of 31 (
The bed bug, Cimex lectularius, shows combination of unusual cytogenetic characteristics, partly common for all Heteroptera. The chromosomes are holokinetic, with completely achiasmatic male meiosis of collochore type and inverted meiosis of the sex chromosomes. A particularly remarkable feature is numerical variation in the number of the sex chromosomes. The standard karyotype of the bed bug contains 26 autosomes and a varying number of supernumerary chromosomes which is supposed to originate after fragmentation of the X chromosome (e.g.
The related species Cimex pipistrelli Jenyns, 1839 is known as an obligate parasite of bats which may share its hosts with Cimex lectularius. The karyotype of Cimex pipistrelli is similar to the standard complement of Cimex lectularius but contains a higher number of autosomes (2n=28+X1X2Y;
The recent expansion is a reason why cytogenetic analysis of this species starts to be more important in respect of recent findings indicating that karyotypic divergences could have evolved faster than DNA sequences (e.g.
This study reports cytogenetic findings in Cimex lectularius and Cimex pipistrelli based on large samples of studied individuals from the Czech Republic and other European countries. We aim to investigate karyotypic variation reported previously in the bedbug and to obtain data revealing possible temporal and geographic pattern of this variation. Another goal of this study is to contribute to better understanding of the mechanisms underlying this variability.
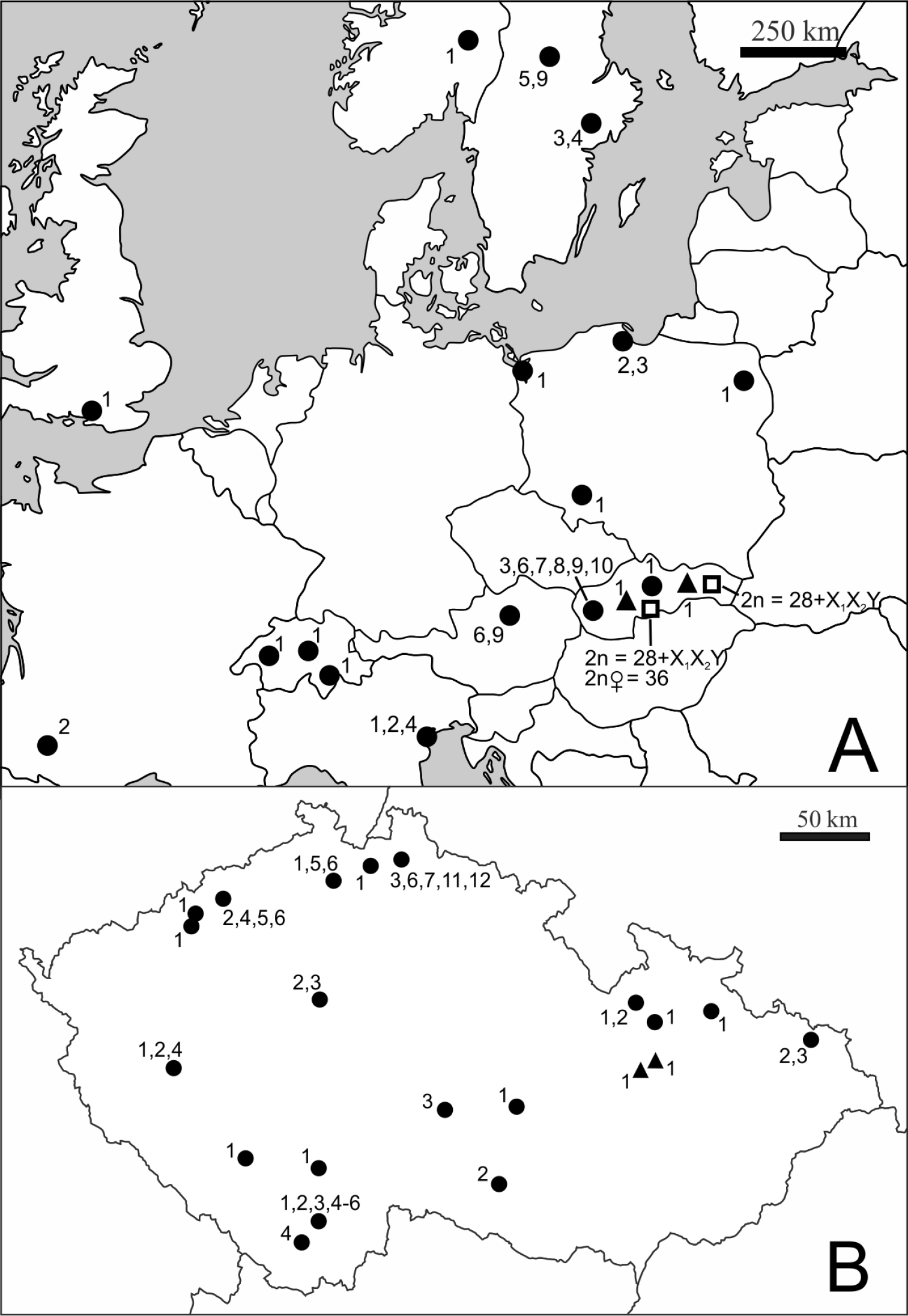
The studied specimens of Cimex lectularius and Cimex pipistrelli were collected from bat roosts and human dwellings in 2010–2012 (Fig. 1). The karyotype was determined in 116 specimens of Cimex lectularius from 61 localities within 10 European countries and in five specimens of Cimex pipistrelli from two localities in Slovakia. The live individuals of synantropic bed bugs from humans were mostly collected by pest exterminators in flats, hotels and hostels. The studied samples originated from individual collecting sites which were localized with varying levels of precision, particularly in the synathropic habitats (flat, house, town, city) depending on information available from the collectors. Individual sites within a single city are differentiated by numerals (e.g., Prague 1, Prague 2). Bugs identified as Cimex lectularius were also collected at four sites of bat roosts in the Czech Republic and Slovakia. The complete list of the collecting sites is shown in Table 1.
Geographical distribution of the sites studied. A Samples of Cimex lectularius and Cimex pipistrelli from Europe B Samples of Cimex lectularius from Czech Republic. ● Cimex lectularius, human habitats, ▲ Cimex lectularius, bat roosts, □ Cimex pipistrelli. Numbers refer to karyotypes 1–12 described in Results.
The list of the collecting sites and a summary of primary results. A = Austria, CH = Switzerland, CZ = Czech Republic, F = France, GB = Great Britain, I = Italy, N = Norway, PL = Poland, S = Sweden, SK = Slovakia. Specimens: left column males, right column females. Designation of the type of karyotype in the last column is the same as in the text and Table 2.
| Sample Code | Country | Locality | Specimens | Karyotype | |
|---|---|---|---|---|---|
| Cimex pipistrelli | ♂ | ♀ | |||
| 190 | SK | Hontianske Nemce | 1 | 2 | see text |
| 191 | SK | Ľubovec | 2 | see text | |
| Cimex lectularius | |||||
| Host: Myotis myotis (Borkhausen, 1797), Myotis emarginatus (E. Geoffroy, 1806) | |||||
| 417 | CZ | Bílá Lhota | 1 | 1 | |
| 418 | CZ | Moravičany | 1 | 1 | |
| 421 | SK | Krásnohorské Podhradie | 2 | 1 | |
| 423 | SK | Hosťovce | 2 | 1 | 1 |
| Cimex lectularius | |||||
| Host: Homo sapiens | |||||
| 609 | CZ | Bruntál | 1 | 1 | |
| 610 | CZ | Plzeň (1) | 2 | 1 | 1 |
| 612 | CZ | Chomutov – Dřínovská | 1 | 1 | 1 |
| 613 | CZ | Liberec (1) – Krejčího | 2 | 1 | 3, 6 |
| 614 | CZ | Liberec (2)- Krejčího | 3 | 7, 11, 12 | |
| 615 | CZ | Jirkov - Na Borku | 1 | 1 | 1 |
| 617 | CZ | Štědrákova Lhota | 1 | 1 | 1, 2 |
| 618 | CZ | Stráž pod Ralskem | 1 | 1 | |
| 619 | CZ | Bohumín – Studentská | 3 | 2, 3 | |
| 621 | CZ | Plzeň (2) – Na Vinicích | 2 | 1 | |
| 623 | CZ | Šumperk | 1 | 1 | 1 |
| 624 | CZ | Plzeň (3) – Na Slovanech | 1 | 1 | 1 |
| 625 | CZ | Plzeň (4) – Na Slovanech | 2 | 1 | 1 |
| 629 | CZ | České Budějovice (1) – Puklicova | 1 | 3 | |
| 632 | CZ | Janov | 1 | 2 | 2, 4, 5, 6 |
| 633 | CZ | Jaroměřice nad Rokytnou | 1 | 2 | |
| 634 | CZ | Plzeň (5) | 1 | 2 | |
| 640 | CZ | Plzeň (6) – Na Slovanech | 2 | 1 | |
| 642 | CZ | Praha (1) | 2 | 2 | |
| 643 | CZ | Praha (2) | 1 | 3 | |
| 644 | CZ | České Budějovice (2) | 2 | 1 | |
| 645 | CZ | České Budějovice (3) - Okružní | 1 | 3 | |
| 647 | CZ | Praha (3) | 1 | 2 | |
| 648 | CZ | Praha (4) | 3 | 2 | |
| 657 | CZ | Plzeň (7) | 2 | 1, 4 | |
| 658 | CZ | Humpolec | 2 | 1 | 3 |
| 659 | CZ | Praha (5) – Křížíkova | 1 | 2 | |
| 661 | CZ | Česká Lípa – Svárovská | 3 | 1 | 1, 5, 6 |
| 662 | CZ | České Budějovice (4) – Netolická | 1 | 1 | 2, 4-6 |
| 665 | CZ | Chvalšiny | 2 | 4 | |
| 667 | CZ | Týn nad Vltavou – Hlinecká | 1 | 1 | |
| 668 | CZ | České Budějovice (5) – J. Bendy | 1 | 1 | |
| 669 | CZ | Strakonice – Bezděkovská | 1 | 1 | |
| 670 | CZ | České Budějovice (6) – M. Chlajna | 2 | 1 | |
| 671 | CZ | Žďár nad Sázavou | 1 | 1 | |
| 707 | SK | Banská Bystrica | 2 | 1 | |
| 708 | SK | Trnava | 5 | 1 | 3, 6, 7, 8, 9, 10 |
| 719 | GB | Brighton | 1 | 1 | |
| 720 | A | Melk | 1 | 2 | 6, 9 |
| 732 | CH | Luzern | 1 | 1 | |
| 737 | CH | - | 1 | 1 | |
| 745 | CH | Fribourg – Rue de l´Hôpital | 1 | 1 | 1 |
| 750 | I | Mestre | 1 | 2 | 2 |
| 751 | I | Venezia (1) | 1 | 1 | |
| 752 | I | Venezia (2) | 2 | 1 | 1, 2 |
| 753 | I | Venezia (3) | 1 | 1, 4 | |
| 789 | N | Ottestad | 1 | 1 | |
| 795 | S | Borlänge (1) | 2 | 5 | |
| 796 | S | Borlänge (2) | 1 | 1 | 5, 9 |
| 798 | S | Stockholm – Vårber | 1 | 2 | 3, 4 |
| 817 | F | Aire/Adour | 2 | 2 | |
| 831 | PL | Świnoujscie | 1 | 1 | |
| 838 | PL | Gdansk (1) | 1 | 2 | |
| 840 | PL | Gdansk (2) | 2 | 1 | 2, 3 |
| 843 | PL | Wroclav – Grabiszynska | 1 | 1 | |
| 844 | PL | Białystok (1) | 1 | 1 | |
| 845 | PL | Białystok (2) | 1 | 1 | |
The chromosome preparations were made from gonads or midgut using the spreading technique described by
After withdrawing of tissues for cytogenetic methods, the material was preserved in 96% ethanol and used in parallel molecular studies. Their results have approved the original specimens determination according to morphological characters (
The karyotype of all the specimens of Cimex lectularius analysed contained 26 autosomes and a varying number of the sex chromosomes. The relative length of chromosomes in the complement was successively diminishing from 5.3 to 1.7%. No distinct size groups of chromosomes could be differentiated; however, the largest and the smallest autosomal pair could be usually recognized according to their size. The original sex chromosomes X1X2Y were medium-sized whereas their supposed fragments occurring in the karyotypes with higher chromosome numbers were the smallest elements of the set.
In the samples of Cimex lectularius studied, 12 distinct karyotypes were differentiated (Table 2). These karyotypes were distinguished according to the varying diploid chromosome number (2n=29–37, 40, 42, 47 in the male complement) and the varying number of the X chromosomes (2–20).
The distribution of samples studied in individual karyotypes characterized in the text. A = Austria, CH = Switzerland, CZ = Czech Republic, F = France, GB = Great Britain, I = Italy, N = Norway, PL = Poland, S = Sweden, SK = Slovakia. Single female possessing the odd number of chromosomes is not included.
| Karyotype | 2n | Sex chromosomes | No. of samples | No. of specimens | Country | ||
|---|---|---|---|---|---|---|---|
| 1 | 29 | 2XY | 35 | 57.4 | 51 | 44.0 | CZ, GB, CH, I, N, PL, SK |
| 2 | 30 | 3XY | 15 | 24.6 | 24 | 20.7 | CZ, F, I, PL |
| 3 | 31 | 4XY | 9 | 14.8 | 13 | 11.2 | CZ, S, SK |
| 4 | 32 | 5XY | 4 | 6.6 | 5 | 4.3 | CZ, S |
| 5 | 33 | 6XY | 3 | 4.9 | 5 | 4.3 | CZ, S |
| 6 | 34 | 7XY | 3 | 4.9 | 3 | 2.5 | A, CZ, SK |
| 7 | 35 | 8XY | 2 | 3.3 | 2 | 1.7 | CZ, SK |
| 8 | 36 | 9XY | 1 | 1.6 | 1 | 0.9 | SK |
| 9 | 37 | 10XY | 3 | 4.9 | 3 | 2.5 | A, S, SK |
| 10 | 40 | 13XY | 1 | 1.6 | 1 | 0.9 | SK |
| 11 | 42 | 15XY | 1 | 1.6 | 1 | 0.9 | CZ |
| 12 | 47 | 20XY | 1 | 1.6 | 1 | 0.9 | CZ |
| mosaic | - | - | 5 | 8.2 | 5 | 4.3 | A, CZ, I, SK |
The identical karyotype was found in all the specimens studied in 46 monomorphic samples, whereas karyotype differences were recorded between individuals in 15 polymorphic samples. We should note, however, that about half of the studied samples (26) consisted of a single specimen only. The results recorded in individual collecting sites are summarized in Table 1.
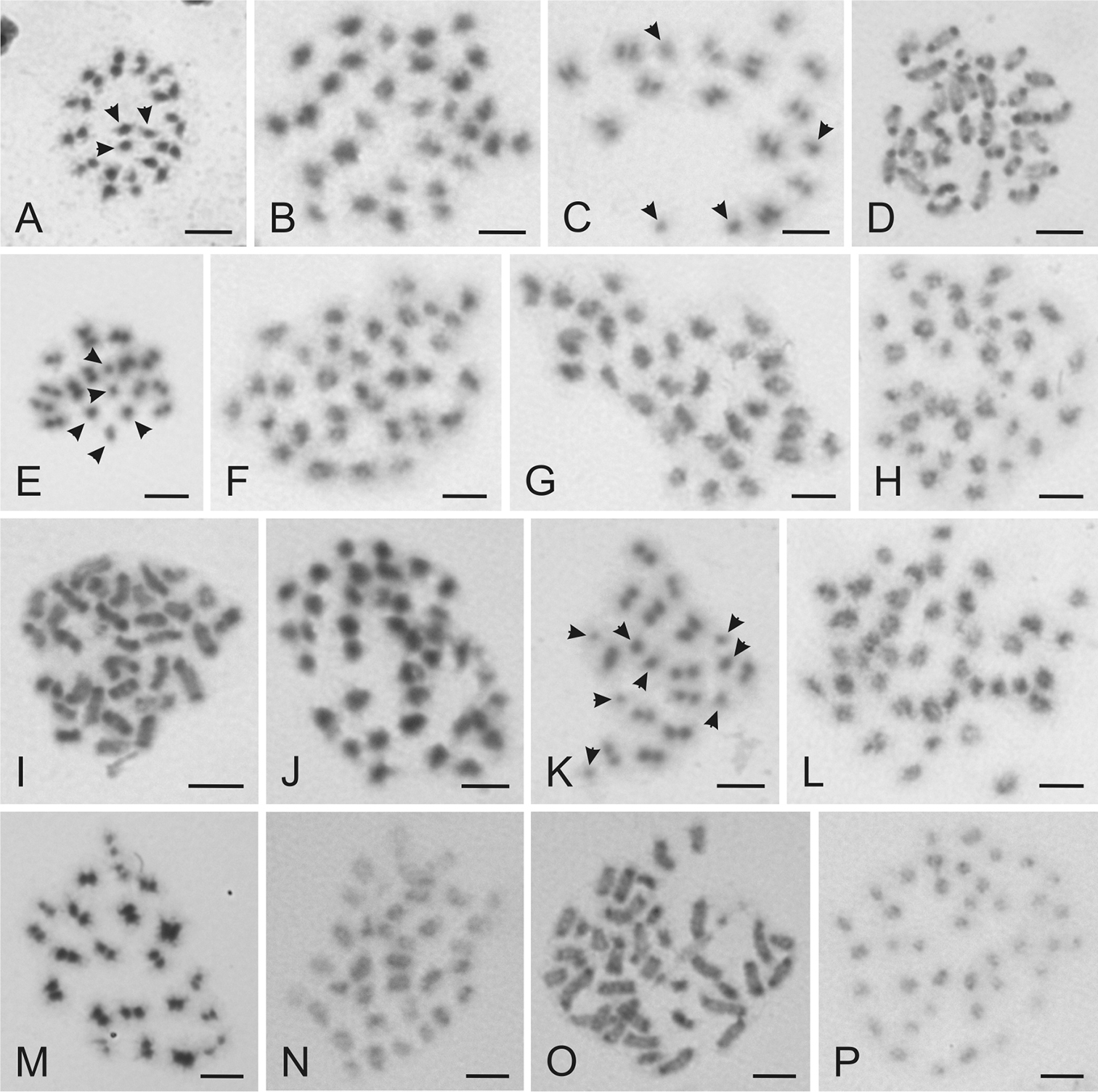
The most common karyotype 1 was characterized by the standard complements with two X chromosomes; 2n=29 in males (2n=26+X1X2Y) and 2n=30 in females (2n=26+X1X1X2X2) (Fig. 2a, b). This complement was found in 51 specimens (33 males and 18 females) and in 31 monomorphic and four polymorphic samples. Seven monomorphous samples of this karyotype included females only. The monomorphic samples from synanthropic habitats were collected in the Czech Republic, Great Britain, Italy, Norway, Poland, Slovakia and Switzerland. This karyotype was further recorded in some individuals from the polymorphic samples collected in the Czech Republic and Italy and in the all samples of Cimex lectularius collected in bat roosts (Fig. 1).
Karyotype 2 included complements with three X chromosomes; 2n=30 in males (2n=26+X1-3Y) and 2n=32 in females (2n=26+2X1-3) (Fig. 2c, d). This chromosome constitution was recognized in 24 specimens (15 males and 9 females) from 15 samples. The karyotype was recorded in both the monomorphic and polymorphic samples. The monomorphic samples from synanthropic habitats in the Czech Republic and Poland included males only, the sample from Italy included males and females, and other samples from the Czech Republic and France included females only. This karyotype was further found in polymorphic samples from the Czech Republic, Italy and Poland.
Karyotype 3 included complements with four X chromosomes; 2n=31 in males (2n=26+X1-4Y) and 2n=34 in females (2n=26+2X1-4) (Fig. 2e, f). This complement was found in 13 specimens (8 males and 5 females) from nine samples. The karyotype was recorded in monomorphic samples from the synathropic habitats collected in the Czech Republic and in polymorphic samples from the Czech Republic, Slovakia and Sweden.
Karyotype 4 included complements with five X chromosomes; 2n=32 in males (2n=26+X1-5Y) and 2n=36 in females (2n=26+2X1-5) (Fig. 2g, h). It was found in five specimens (2 males and 3 females) from four samples. This complement was recorded in a monomorphic sample from the Czech Republic and in polymorphic samples from the Czech Republic and Sweden.
Karyotype 5 included complements with six X chromosomes; 2n=33 in males (2n=26+X1-6Y) and 2n=38 in females (2n=26+2X1-6) (Fig. 2i, j) and it was found in five specimens (4 males and 1 female) from three samples. This complement was identified in a single monomorphic sample including two males collected in Sweden and in polymorphic samples from the Czech Republic and Sweden.
Karyotype 6 included complements with seven X chromosomes; 2n=34 in males (2n=26+X1-7Y) and 2n=40 in females (2n=26+2X1-7) (Fig. 2k, l) and it was found in three specimens (1 male and 2 females) from three polymorphic samples collected in Austria and the Czech Republic.
Karyotype 7 included a complement with eight X chromosomes and 2n=35 in males (2n=26+X1-8Y) (Fig. 2m) and it was found in two male specimens collected in polymorphic samples from two sites in the Czech Republic and Slovakia, respectively.
Karyotype 8 included a complement with nine X chromosomes and 2n=36 in males (2n=26+X1-9Y) (Fig. 2n) and it was recorded in a single male collected in the Slovakia.
Karyotype 9 included a complement with ten X chromosomes and 2n=37 in males (2n=26+X1-10Y) (Fig. 2o) and it was recorded in three males collected in the Austria, Slovakia and Sweden.
Karyotype 10 included a complement with 13 X chromosomes and 2n=40 in males (2n=26+X1-13Y) (Fig. 2p). This karyotype was identified in a single male collected in Slovakia.
Examples of chromosomes of Cimex lectularius from various stages of cell division stained with Giemsa. A Metaphase II ♂, 2n=29 B Mitotic metaphase ♀, 2n=30 C Metaphase II ♂, 2n=30 D Mitotic prometaphase ♀, 2n=32 E Metaphase II ♂, 2n=31 F Mitotic metaphase ♀, 2n=34 G Mitotic metaphase ♂, 2n=32 H Mitotic metaphase ♀, 2n=36 I Mitotic prometaphase ♂, 2n=33 J Mitotic metaphase ♀, 2n=38 K Metaphase II ♂, 2n=34 L Mitotic metaphase ♀, 2n=40 M Metaphase I ♂, 2n=35 N Mitotic metaphase ♂, 2n=36 O Mitotic prometaphase ♂, 2n=37 P Mitotic metaphase ♂, 2n=40. Arrows indicate sex chromosomes. Bar = 5 μm.
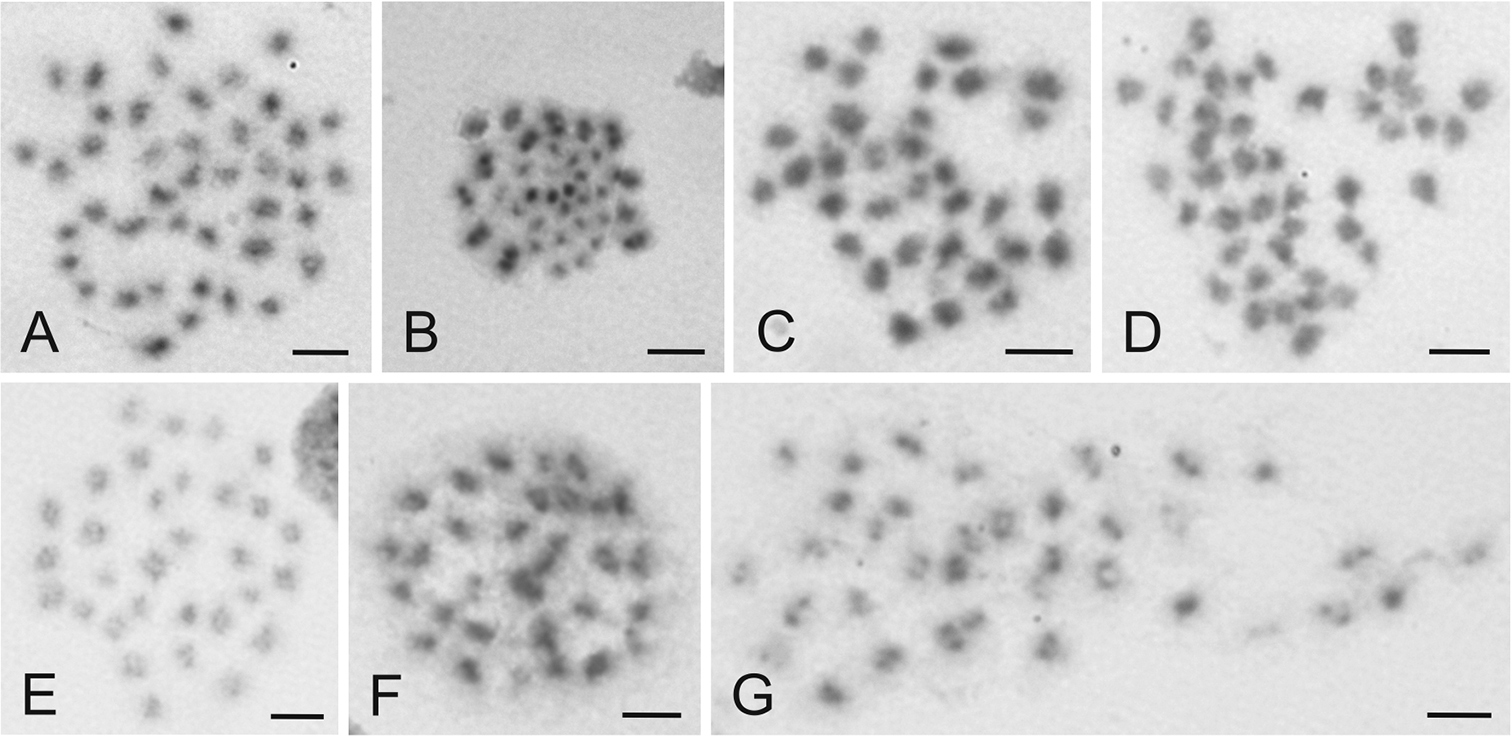
Karyotype 11 included a complement with 15 X chromosomes and 2n=42 in males (2n=26+X1-15Y) (Fig. 3a). This karyotype was identified in a single male collected in the Czech Republic.
Karyotype 12 included complements with 20 X chromosomes and 2n=47 in males (2n=26+X1-20Y) (Fig. 3b). This complement was identified in a single male from the Czech Republic.
The females exhibited the number of chromosomes which was usually complementary to the number established in the males from the same sample. However, 11 polymorphic samples were revealed in which the karyotypes of females and males were not complementary one another. Two females showing karyotypes with odd numbers of X chromosomes (7 and 17; 2n=33 and 43, respectively) were recorded (Fig. 3c, d).
Examples of chromosomes of Cimex lectularius (A–D) and Cimex pipistrelli (E-G) from various stages of cell division stained with Giemsa. A Mitotic metaphase ♂, 2n=42 B Metaphase II ♂, 2n=47 C Mitotic metaphase ♀, 2n=33 D Mitotic metaphase ♀, 2n=43 E Mitotic metaphase ♂, 2n=31 F Mitotic metaphase ♀, 2n=32 G Mitotic metaphase ♀, 2n=36. For more details see text. Bar = 5 μm.
The occurrence of chromosomal mosaics with the karyotype constitution varying between cells of single individual was observed in five specimens (2 males and 3 females) from five samples. A female from Slovakia (Trnava) had two karyotypically different cell types. The complement with 14 X chromosome fragments (2n=40) was found in mesenteron cells, whereas 17 X chromosome fragments (2n=43) were observed in germinal cells from ovarium. In other individuals showing mosaic, variation was recorded between germinal cells derived from gonads only. In a single female from Austria (Melk), the karyotypes with 12 and 14 X chromosome fragments were recorded (2n=38 and 40, respectively). A male from Janov in the Czech Republic showed cells with six or seven X fragments (2n=33 and 34, respectively). A similar mosaic constitution was recorded in a female from České Budějovice (4) (2n=36 and 40, respectively). Mosaicism with two and five X chromosome fragments was also revealed in a male from Italy (Venezia 3) (2n=29 and 32, respectively).
The karyotypes with higher chromosome numbers as well as individuals with chromosomal mosaic were usually found within the samples exhibiting particularly extensive variation between individuals. A sample from Liberec contained two males with karyotypes 2n=26+X1-4Y and a single female with 2n=26+2X1-14. The other sample from Liberec, collected in another flat in the same house, included three males with distinctly different karyotypes (2=26+X1-8Y, 2n=26+X1-15Y, 2n=26+X1-20Y). The Trnava sample contained five males with different karyotypes (2n=26+X1-4Y, 2n=26+X1-8Y, 2n=26+X1-9Y, 2n=26+X1-10Y, 2n=26+X1-13Y) and a female showing a mosaic karyotype with different chromosomal numbers observed in both examined tissues. The Melk sample included a male with 2n=26+X1-10Y, and two females, one with 2n=26+2X1-7 and another with a mosaic karyotype constitution 2n=26+2X1-12/14.
The karyotype of Cimex pipistrelli included 28 autosomes and the sex chromosome trivalent X1X2Y (males 2n=28+X1-2Y=31, females 2n=28+2X1-2=32; Fig. 3e, f). This complement was found in four specimens examined. The complement of a female from Slovakia (Hontianske Nemce) contained eight X chromosomes (2n=28+2X1-4=36; Fig. 3g).
Our data confirm considerable variation in the karyotype of the bed bug and further extend its range (Table 3). The distribution of the karyotypes in various Czech and European localities appeared random, and did not show any consistent geographic pattern. Therefore, no reliable information concerning the historical or current dispersal of bed bugs can be derived.
A synopsis of known karyotypes inCimex lectularius. References: 1 -
| Karyotype | X, Y | 2n | Country | References |
|---|---|---|---|---|
| 1 | 2XY | 29 | CH, CZ, BG, F, GB, I, J, MEX, N, PL, RUS, SK, USA | 1, 3, 4, 5, this study |
| 2 | 3XY | 30 | CZ, F, GB, I, PL | 1, 2, this study |
| 3 | 4XY | 31 | CZ, GB, S, SK | 1, 2, this study |
| 4 | 5XY | 32 | CZ, GB, I, PL, S | 1, 2, this study |
| 5 | 6XY | 33 | CZ, ET, GB, PL, S, USA | 1, 2, 3, this study |
| 6 | 7XY | 34 | A, CZ, GB, PL, SK, USA | 1, 2, 3, this study |
| 7 | 8XY | 35 | CZ, GB, SK, USA | 1, 2, 3, this study |
| 8 | 9XY | 36 | GB, SK, USA | 1, 2, 3, this study |
| 9 | 10XY | 37 | A, GB, S, SK | 1, 2, this study |
| 10 | 11XY | 38 | GB | 1, 2 |
| 11 | 12XY | 39 | GB | 1, 2 |
| 12 | 13XY | 40 | GB, SK | 1, 2, this study |
| 13 | 14XY | 41 | GB | 1, 2 |
| 14 | 15XY | 42 | CZ, GB | 2, this study |
| 15 | 20XY | 47 | CZ | this study |
We have obtained certain findings that are at variance with the previously published results. The distribution pattern of incidence of the X chromosome fragments reported by
The results obtained in Cimex pipistrelli confirm the previously published data (
There are various possible explanations of the origin of extensive variation in the chromosome number in the karyotypes of bed bugs. The elements responsible for numerical variation in bed bugs could belong to a specific chromosomal type known in other heteropterans. In 14 families of this order, a special pair of chromosomes occurs called the m-chromosomes (e.g.
B chromosomes were reported in species from various bug families including Cimicidae (
Therefore, the most plausible explanation of the origin of the supernumerary elements in the bed bug complements remains fragmentation of the X chromosome. This mechanism produces a complicated system of multiple sex chromosomes and it was proposed already in previously published papers (see
The causes of the origin and maintenance of extensive fragmentation of the X chromosome of bed bugs remain unclear. Populations of bedbugs have been exposed to various insecticides all over the world for decades (
We found an extraordinarily wide extent of karyotype variation between specimens in a few population samples only. We assume that this extreme variation might result from random mixing of individuals of different origin at a single site. Mating between geographically unrelated individuals can easily be imagined in a parasite such as the bed bug transmitted by migrating people. However, it is difficult to explain why these highly variable samples usually included specimens with an extreme karyotype constitution and the highest numbers of the X chromosome fragments. It is obvious that mating of parents with different karyotypes can produce great variety of recombinant complements in offspring, particularly in females. Variation in the number of chromosome fragments may be associated with abnormalities occurring in chromosome segregation during the cell division. The regular course of meiosis in the bed bug may be influenced by the holokinetic nature of chromosomes, completely achiasmatic male meiosis and inverted meiosis of the sex chromosomes.
We can only speculate about relationships between the system of transmission of the fragmented sex chromosomes and the unusual features of reproductive biology of bed bugs. The assumed lowering of the fitness of individuals carrying higher numbers of the X chromosome fragments could potentially affect population dynamics of variable populations. It is apparent that more intensive cytogenetic screening combined with data on molecular variation in DNA sequences might shed light to this question.
Our special thanks to be expressed to many specialists and colleagues who have taken part in collecting live bed bug material for the study, without their great help the study cannot be done.
We are greatly obliged to all colleagues who helped us with sampling of bed bugs: A. Drozda, P. Dvořák, P. Foltán, S. Kováč, M. Kučera, V. Měřínský, M. Novotný, L. Pěček, V. Prchal, S. Ritterová, P. Sodomek, R. Šimák, M. Toman, J. Vondráčková and J. Zelená (Czech Republic). S. Boscolo (Italy), Ł. Brożek (Poland), L. Duplantier (France), T. Hutson (Great Britain), A. Larson (Sweden), H. Kjellberg (Sweden), E. Krug (Switzerlnand), M. Kadej (Poland), M. Schmidt (Switzerland), P. Szewczyk (Poland) and M. Wegman (Switzerland). We appreciate also great help of pest control managers from Biotech Salzburg (Austria), DDD servis (Czech Republic), Ekolas (Slovakia), Pelias Norsk Skadedyrkontroll (Norway) and Verminex (Poland).
Special thanks is due to O. Balvín (Charles University, Prague) with whom the all study was coordinated, particularly concerning material. We thank to M. Forman and J. Král (Charles University, Prague) for their help with lab work and interpretation of photographs. We are indebted to F. Marec (Biology Centre AS CR, České Budějovice) and P. Ráb (Institute of Animal Physiology and Genetics AS CR, Liběchov) for comments to the study and text.
The study was supported by the grants of Ministry of Education, Youth and Sports of the Czech Republic no. SVV/2013-267 201 and the Grant Agency of Charles University no. 267111 B-BIO/2011.