Citation: Turco A, Medagli P, Albano A, D’Emerico S (2014) Karyomorphometry on three polyploid species of Arum L. (Araceae, Aroideae). Comparative Cytogenetics 8(1): 71–80. doi: 10.3897/CompCytogen.v8i1.6444
In this study three polyploid Arum Linnaeus, 1753 species from Southern Italy were chromosomally investigated. Arum italicum Miller, 1768 was found to have 2n = 84 chromosomes and a karyotype composed of numerous asymmetric chromosomes. Arum maculatum Linnaeus, 1753 and Arum apulum (Carano) P. C. Boyce, 1993 were found to have 2n = 56 chromosomes. In the examined taxa some chromosome pairs were characterized by the presence of weakly coloured Feulgen-stained segments. The karyotype morphology of Arum italicum was found to be similar to that of Arum maculatum, but the more asymmetrical karyotype and numerous weakly coloured Feulgen-stained segments observed in the former suggest the existence of more extensive rearrangements. In contrast, Arum apulum was observed to have a symmetrical karyotype. The A1, A2 and SYi karyotype asymmetry indices are presented. The relationships between these taxa in terms of karyotype morphology and evolution are discussed.
Allocyclic segments, karyotype asymmetry, karyotype evolution, Arum apulum, Arum italicum, Arum maculatum
The high biodiversity of Araceae Jussieu, 1789, with ca. 109 genera and over 3700 species (
In this study we conducted a karyomorphometric survey of Arum Linnaeus, 1753, a small herbaceous genus containing about 28 species (
From a karyological point of view, the basic number for the Arum genus is x = 14 (
Cytological investigations of Arum chromosome numbers have sought to clarify its taxonomy (
The purpose of this study is to acquire detailed new information on the karyomorphometry and chromosome structure of Arum italicum, Arum maculatum, and Arum apulum from Southern Italy.
Samples of Arum italicum were collected from various sites in Puglia and Lucania, while samples of Arum maculatum were collected near Muro Lucano - Potenza (Lucania) and Arum apulum near Quasano, Sammichele, Turi - Bari (Puglia) (Table 1). Only Arum apulum and Arum italicum are cultured in the Museo Orto Botanico di Bari (Bari). The nomenclature used for classification follows
Arum taxa investigated and origin of samples.
| Taxon | Locality | Collector |
|---|---|---|
| Arum apulum | Apulia: Quasano (Bari) | Medagli and D’Emerico 13.IV.2010 |
| Apulia: Sammichele (Bari) | Medagli and D’Emerico 15.IV.2010 | |
| Apulia: Turi (Bari) | Medagli and D’Emerico 15.IV.2010 | |
| Arum italicum | Apulia: Quasano (Bari) | Medagli and D’Emerico 13.IV.2010 |
| Apulia: Sammichele (Bari) | Medagli and D’Emerico 15.IV.2010 | |
| Apulia: Turi (Bari) | Medagli and D’Emerico 15.IV.2010 | |
| Lucania: Matera | Medagli and D’Emerico 22.IV.2010 | |
| Lucania: Grottole (Matera) | Medagli and D’Emerico 23.IV.2010 | |
| Lucania: Pomarico (Matera) | Medagli and D’Emerico 23.IV.2010 | |
| Arum maculatum | Lucania: Muro Lucano (Potenza) | Medagli and D’Emerico 27.V.2010 |
Root-tips were pretreated in 0.3% aqueous colchicine at 20°C for two hours, and subsequently fixed for five min in a 5:1:1:1 (volume ratio) mixture of absolute ethanol, chloroform, glacial acetic acid and formalin. Hydrolysis was carried out at 20°C in 5.5 N HCl for 20 min (Battaglia 1957 a, b), then stained with Schiff’s reagent. Root tips were squashed in a drop of 45% acetic acid.
The nomenclature used for describing karyotype composition followed
For Giemsa C-banding, a modification of
This study provides new cytological information on three polyploid Arum taxa. The present analysis is in agreement with the sectional segregation based on tuber structure in the classification of the Arum genus suggested by
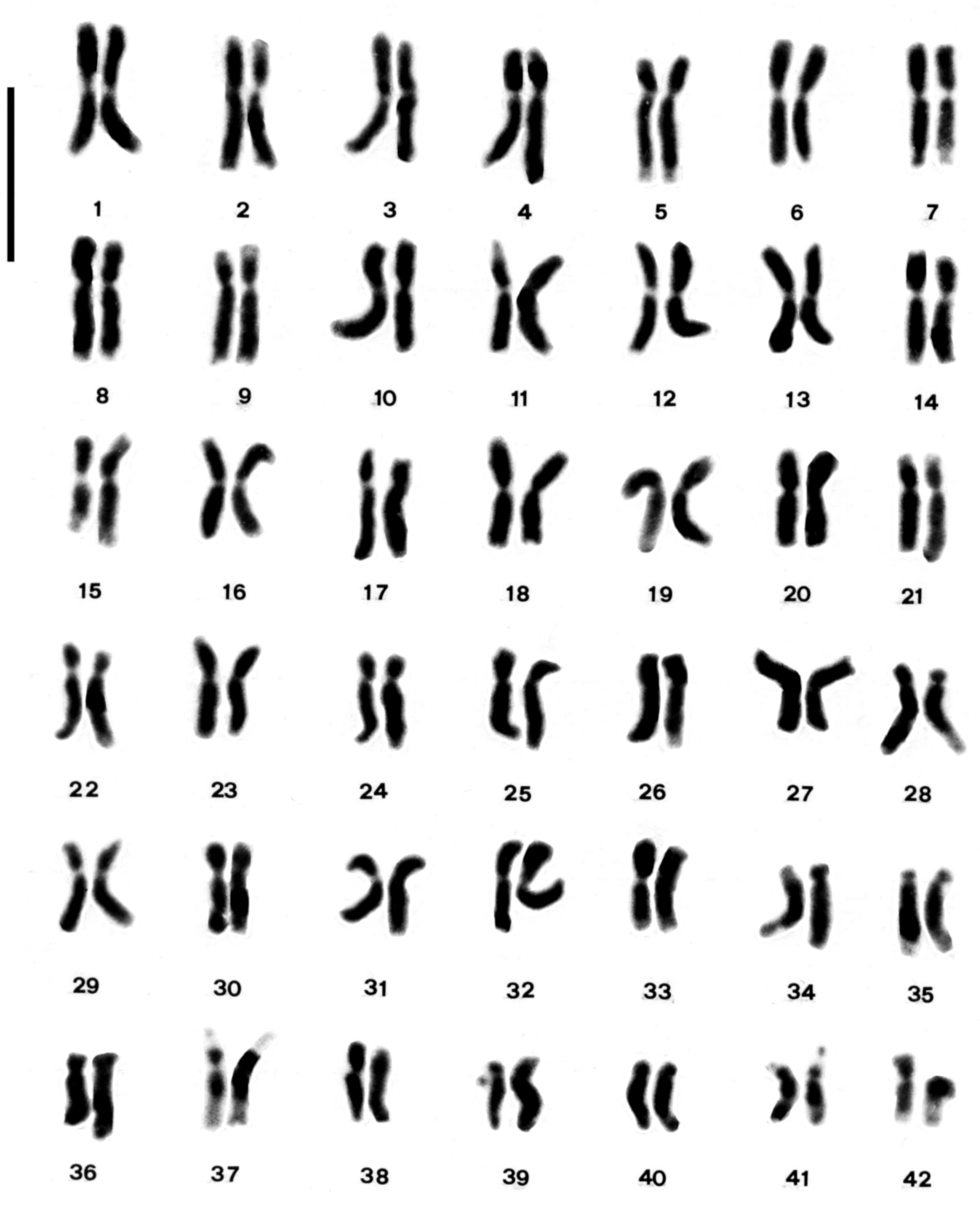
In Arum italicum the chromosome number 2n = 84 (Fig. 2a) was observed in all the investigated populations, which is consistent with previous reports (
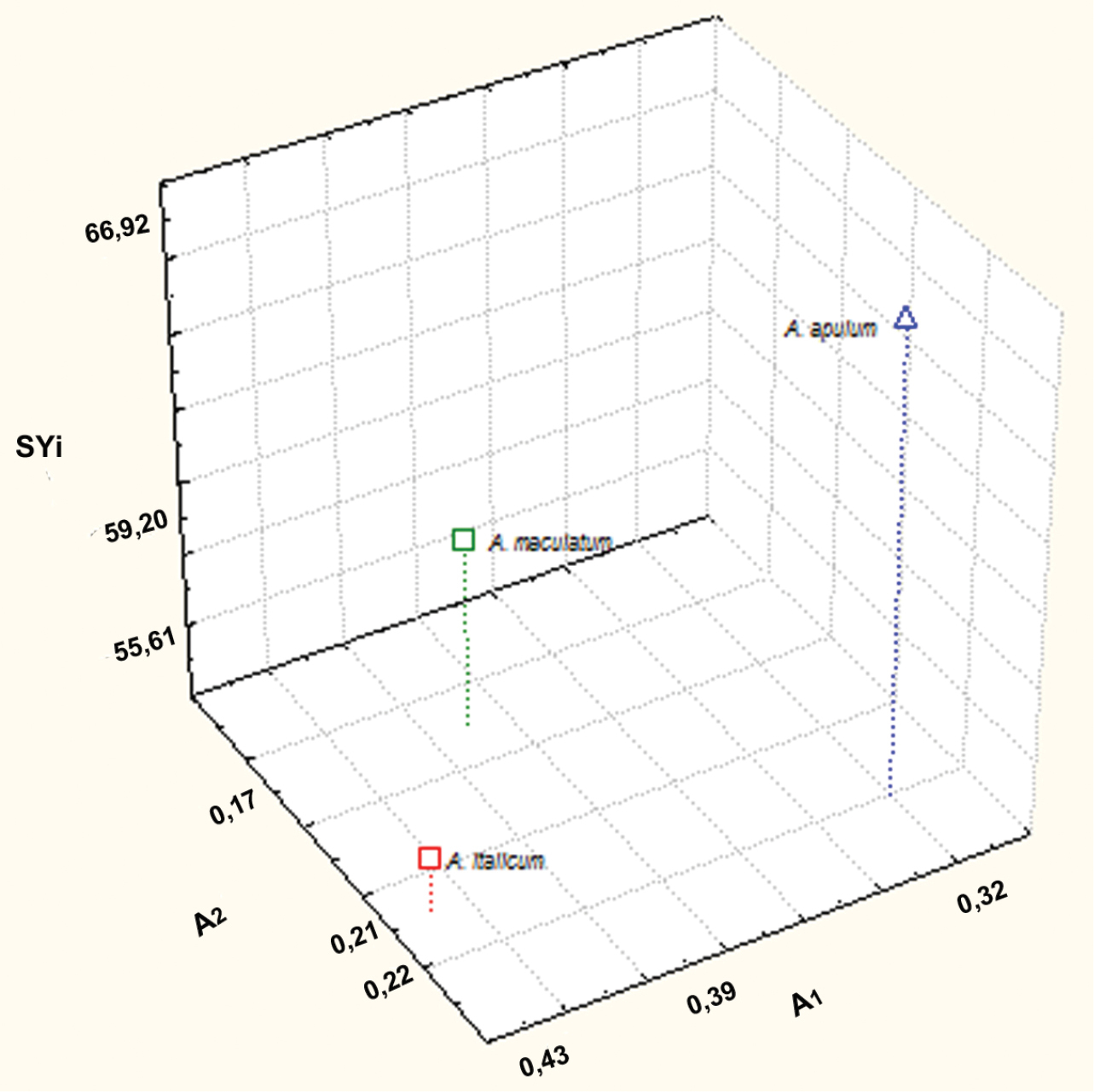
Scatter diagram of A1, A2 and SYi values of Arum taxa examined.
Somatic chromosomes of Arum species: a Arum italicum (2n = 84) b Arum maculatum (2n = 56) c Arum apulum (2n = 56). (Arrows show chromosomes with weakly coloured Feulgen-stained segments) Bar = 5µm.
Karyotype of Arum italicum. Bar = 5µm.
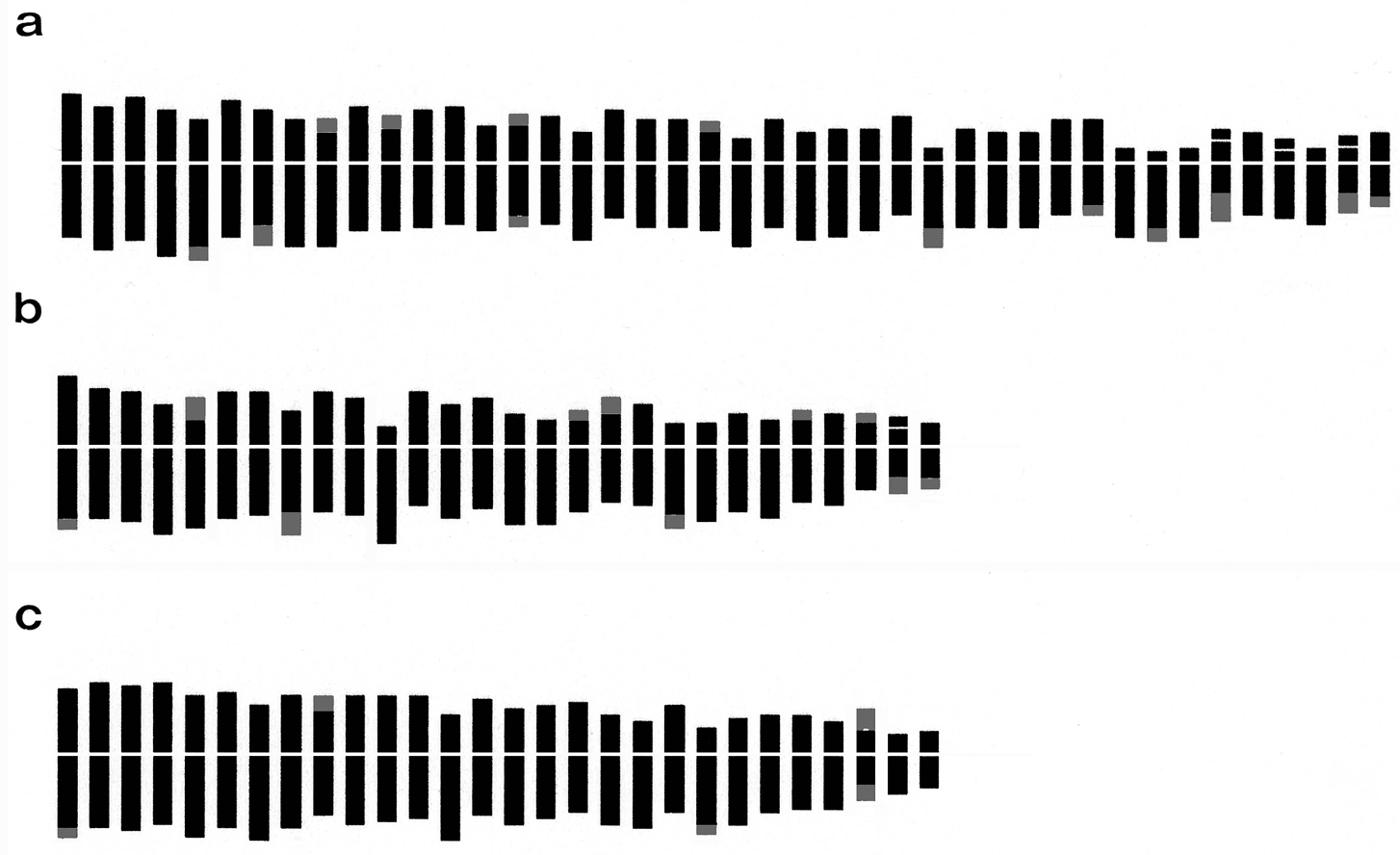
Haploid idiograms of Arum species: a Arum italicum b Arum maculatum c Arum apulum. (Telomeres shaded in gray show chromosomes with allocyclic segments).
Arum maculatum was found to have 2n = 56 somatic chromosomes (Fig. 2b), confirming earlier counts for this species on samples from the Balkan Peninsula (
The samples of Arum apulum from Quasano, Sammichele and Turi (Bari) showed 2n = 56 chromosomes (Fig. 2c), in agreement with previous reports (
The karyotype morphology of Arum italicum is similar to that of Arum maculatum. Arum italicum shows a more asymmetrical karyotype, with a higher intrachromosomal asymmetry index (A1 = 0.43) than Arum maculatum (A1 = 0.39). By contrast, Arum apulum possesses the most symmetrical karyotype of the three (A1 = 0.32) (Fig. 1, Table 2), being composed of mainly metacentric chromosomes and having few allocyclic segments. According to
Morphometric parameters (mean ± S. E.) of the karyotypes of three Arum taxa studied. Haploid complement length; Chromosome number; A1, A2 (Romero Zarco 1986) and Syi (
| Taxa | Haploid complement (µm) | Chromosome number 2n | A1 | A2 | SYi |
| Arum apulum | 90.58 (± 3.12) | 56 | 0.32 (± 0.01) | 0.22 (± 0.01) | 66.92 (± 1.61) |
| Arum maculatum | 96.63 (± 2.46) | 56 | 0.39 (± 0.01) | 0.17 (± 0.01) | 59.20 (± 0.27) |
| Arum italicum | 169.22 (± 16.36) | 84 | 0.43 (± 0.02) | 0.21 (± 0.02) | 55.61 (± 1.90) |
In all the examined taxa some chromosome pairs are characterized by the presence of weakly stained segments, formerly described as secondary constrictions (