Citation: Gokhman VE, Yefremova ZA, Yegorenkova EN (2014) Karyotypes of parasitic wasps of the family Eulophidae (Hymenoptera) attacking leaf-mining Lepidoptera (Gracillariidae, Gelechiidae). Comparative Cytogenetics 8(1): 31–41. doi: 10.3897/CompCytogen.v8i1.6537
Karyotypes of eleven parasitoid species of the family Eulophidae were examined, namely, Chrysocharis laomedon (Walker, 1839) (2n = 10), Chrysocharis sp. aff. laomedon (n = 5, 2n = 10), Chrysocharis sp. aff. albipes (Ashmead, 1904) (2n = 12), Mischotetrastichus petiolatus (Erdös, 1961) (n = 6, 2n = 12), Minotetrastichus frontalis (Nees, 1834) (n = 5, 2n = 10), Cirrospilus pictus (Nees, 1834) (2n = 12), Hyssopus geniculatus (Hartig, 1838) (2n = 16), Sympiesis gordius (Walker, 1839) (2n = 12), S. sericeicornis (Nees, 1834) (2n = 12), Pnigalio agraules (Walker, 1839) (2n = 12 + 0–2B) and Pnigalio gyamiensis Myartseva & Kurashev, 1990 (2n = 12 + 0–6B) reared from Phyllonorycter acerifoliella (Zeller, 1839), Ph. apparella (Herrich-Schäffer, 1855), Ph. issikii (Kumata, 1963) (Gracillariidae) and Chrysoesthia sexguttella (Thunberg, 1794) (Gelechiidae). Chromosome sets of all species except P. agraules and P. gyamiensis were studied for the first time. B chromosomes were detected in the two latter species; in P. gyamiensis, the maximum number of B chromosomes represents the highest value known for parasitic wasps to date.
Hymenoptera, Chalcidoidea, Eulophidae, Lepidoptera, Gracillariidae, Gelechiidae, chromosomes, karyotypes, B chromosomes
The Eulophidae are one of the largest and most diverse families of the hymenopteran superfamily Chalcidoidea. This group currently contains about 300 genera and 4500 described species (
The material used in this study was collected by V.E. Gokhman and E.N. Yegorenkova in the Moscow (Ozhigovo, 60 km SW Moscow; 55°27'N, 36°52'E) and Ulyanovsk Provinces (Ulyanovsk; 54°16'N, 48°20'E) of Russia in 2012–2013 respectively (Table 1). All parasitoids were reared from Phyllonorycter acerifoliella (Zeller, 1839) on Acer platanoides Linnaeus, Phyllonorycter apparella (Herrich-Schäffer, 1855) on Populus tremula Linnaeus, Phyllonorycter issikii (Kumata, 1963) (Gracillariidae) on Tilia cordata Miller and Chrysoesthia sexguttella (Thunberg, 1794) (Gelechiidae) on Chenopodium album Linnaeus. Preparations of mitotic chromosomes (as well as meiotic chromosomes where available) of all species except Minotetrastichus frontalis were obtained from ovaries of adult females according to the protocol developed by
The main results of karyotypic study of the Eulophidae (Hymenoptera) attacking leaf-mining Lepidoptera.
| Species | Origin | Number | (n), 2n | Chromosomal formula: (n), 2n | ||
|---|---|---|---|---|---|---|
| Locality | Host | (Males), females | (Meiotic), mitotic divisions | |||
| Chrysocharis laomedon | Ozhigovo | Phyllonorycter issikii | 1 | 11 | 10 | 10M |
| Chrysocharis sp. aff. laomedon | Ozhigovo, Ulyanovsk | Phyllonorycter acerifoliella, Phyllonorycter issikii | 7 | (3), 13 | (5), 10 | 10M |
| Chrysocharis sp. aff. albipes | Ozhigovo | Phyllonorycter apparella | 1 | 10 | 12 | 10M + 2ST |
| Mischotetrastichus petiolatus | Ditto | Phyllonorycter issikii | 2 | (4), 2 | (6), 12 | 10M + 2A |
| Minotetrastichus frontalis | Ulyanovsk | Ditto | (1), 7 | 15 | (5), 10 | (5M), 10M |
| Cirrospilus pictus | Ozhigovo | Phyllonorycter apparella | 2 | 6 | 12 | 6M + 2SM + 4ST |
| Hyssopus geniculatus | Ditto | Phyllonorycter issikii | 3 | 14 | 16 | 6M + 2SM + 4ST + 4A |
| Sympiesis gordius | Ditto | Ditto | 2 | 2 | 12 | 12M |
| Sympiesis sericeicornis | Ditto | Phyllonorycter apparella | 2 | 10 | 12 | 10M + 2A |
| Pnigalio agraules | Ditto | Phyllonorycter apparella, Phyllonorycter issikii | 3 | 13 | 2n = 12 + 0–2B | 10M + 2M/SM |
| Pnigalio gyamiensis | Ulyanovsk | Chrysoesthia sexguttella | 1 | 17 | 2n = 12 + 0–6B | 6M + 4M/SM + 2ST |
Relative lengths (RL) and centromeric indices (CI) of Pnigalio agraules and Pnigalio gyamiensis chromosomes (mean ± SD; B chromosomes not included). For each species, numbers of analyzed metaphase plates are given in brackets.
| Chromosome no. | Pnigalio agraules (6) | Pnigalio gyamiensis (8) | ||
|---|---|---|---|---|
| RL | CI | RL | CI | |
| 1 | 21.09 ± 1.09 | 44.74 ± 3.50 | 20.44 ± 0.75 | 45.43 ± 3.37 |
| 2 | 18.89 ± 0.84 | 43.32 ± 3.56 | 19.06 ± 0.85 | 40.87 ± 5.13 |
| 3 | 17.31 ± 0.70 | 41.82 ± 3.69 | 18.04 ± 0.54 | 41.40 ± 4.59 |
| 4 | 16.06 ± 0.61 | 44.40 ± 4.63 | 16.69 ± 0.76 | 44.82 ± 3.79 |
| 5 | 14.37 ± 0.83 | 45.18 ± 3.92 | 14.18 ± 0.93 | 45.50 ± 4.61 |
| 6 | 12.28 ± 1.42 | 38.39 ± 5.36 | 11.59 ± 1.24 | 18.82 ± 5.34 |
The principal results of the present study are listed in Table 1; some additional details are given below.
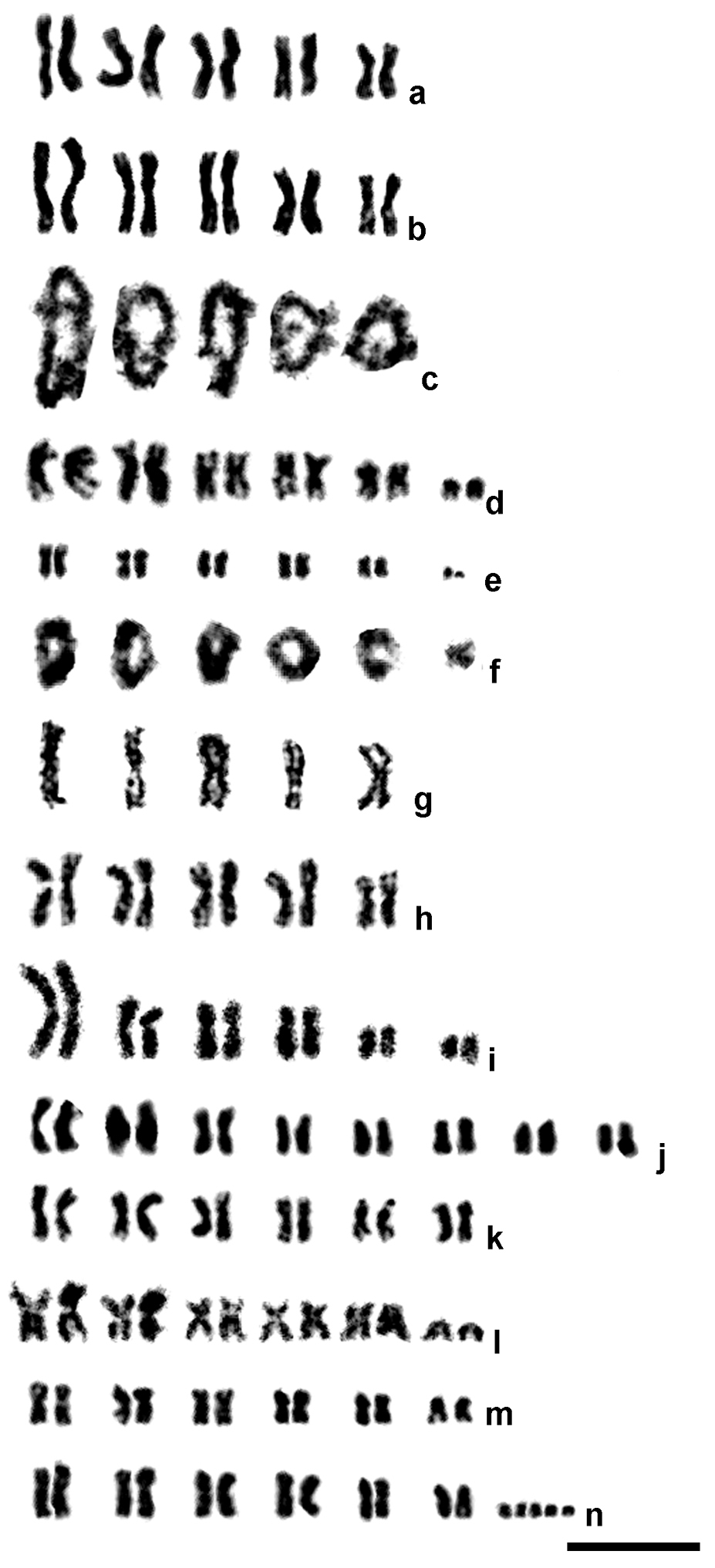
Chrysocharis laomedon (Walker, 1839) (Fig. 1a). All chromosomes are obviously metacentric; chromosomes of the first, second and third pair, and those of the fourth and fifth pair, form three size groups.
Mitotic (a–b, d–e, g–n) and meiotic (diplotene; c, f) karyograms of male (g) and female (a–f, h–n) parasitic wasps of the family Eulophidae. a Chrysocharis laomedon b–c Chrysocharis sp. aff. laomedon d Chrysocharis sp. aff. albipes e–f Mischotetrastichus petiolatus g–h Minotetrastichus frontalis i Cirrospilus pictus j Hyssopus geniculatus k Sympiesis gordius l Sympiesis sericeicornis m Pnigalio agraules n Pnigalio gyamiensis, karyotype with five B chromosomes. Bar = 10 µm (6.7 µm for c).
Chrysocharis sp. aff. laomedon (Fig. 1b–c). Karyotype structure of the mitotic chromosome set as in Chrysocharis laomedon (Fig. 1b). The meiotic karyotype contains five bivalents; each of them apparently bears two chiasmata in diplotene (Fig. 1c).
Chrysocharis sp. aff. albipes (Ashmead, 1904) (Fig. 1d). As in the two previous species, chromosomes of the five largest pairs are metacentric, but a pair of small subtelocentrics is present in the karyotype as well. The metacentrics also form three size groups; however, apart from previous species, these groups include chromosomes of the first and second, third and fourth, and fifth pair respectively.
Mischotetrastichus petiolatus (Erdös, 1961) (Fig. 1e–f). The karyotype contains five pairs of metacentric chromosomes; the first and the last pair are visibly longer/shorter respectively than the remaining ones. In addition, a pair of small acrocentrics is present in the chromosome set (Fig. 1e). Six bivalents are found in the meiotic karyotype of this species; in diplotene, almost all of them bear two chiasmata except for the last one with a single chiasma (Fig. 1f).
Minotetrastichus frontalis (Nees, 1834) (Fig. 1g–h). Both haploid (Fig. 1g) and diploid karyotypes (Fig. 1h) were studied. All chromosomes are obviously metacentric; those of the fifth pair are substantially smaller than chromosomes of the preceding ones.
Cirrospilus pictus (Nees, 1834) (Fig. 1i). Metacentrics of the first pair very large, at least more than 1.5 times longer than the remaining chromosomes. Metacentrics of the second and third pair as well as submetacentrics of the fourth pair more or less gradually decrease in length. Subtelocentric chromosomes of the fifth and sixth pair substantially differ in size and visibly smaller than the preceding ones.
Hyssopus geniculatus (Hartig, 1838) (Fig. 1j). The diploid chromosome number in this species is substantially higher than in many other members of the family. First three chromosome pairs obviously differ in size and are somewhat longer than the remaining ones. The karyotype contains metacentric (the first, third and eighth pair), submetacentric (the second pair), subtelocentric (the fourth and sixth pair) and acrocentric chromosomes (the fifth and seventh pair).
Sympiesis gordius (Walker, 1839) (Fig. 1k). All chromosomes are metacentric; metacentrics of the first pair substantially differ in size from the remaining ones. Chromosomes of the fifth pair bear distinct secondary constrictions.
Sympiesis sericeicornis (Nees, 1834) (Fig. 1l). The karyotype contains five pairs of large metacentric chromosomes and a small pair of acrocentrics; chromosomes of the first pair are visibly longer than the other metacentrics.
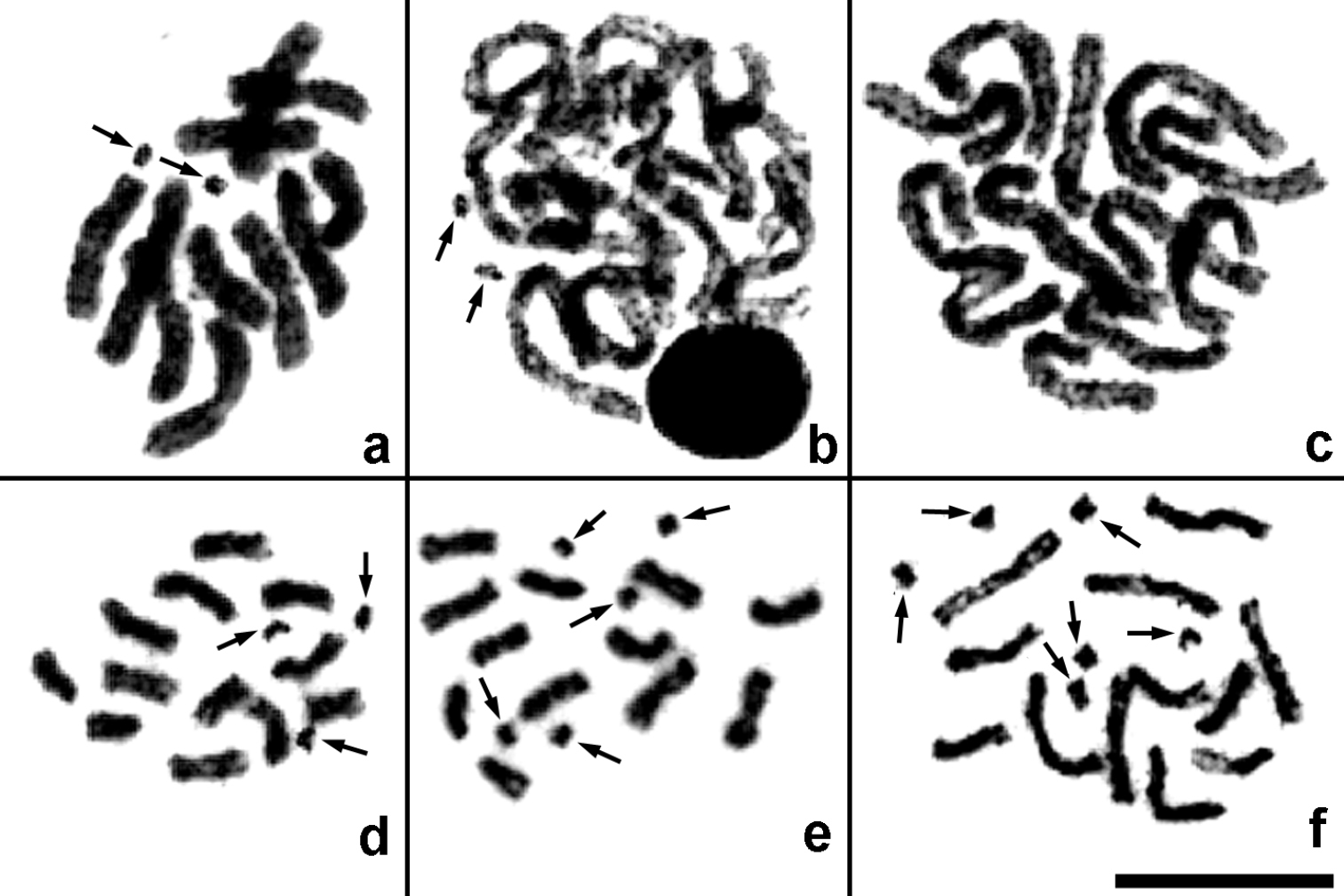
Pnigalio agraules (Walker, 1839) (Figs 1m, 2a–c). The first and the last chromosome pair are obviously longer/shorter respectively than the remaining ones that form a continuous gradation in length (Table 2). Most chromosomes are metacentric except for the sixth pair that can be either metacentric or submetacentric (Fig. 1m). In addition, a single specimen carrying B chromosomes was found. Although we were unable to obtain full metaphase plates in the former individual, fragments of these plates (Fig. 2a) as well as certain mitotic divisions in late prophase or early metaphase (Fig. 2b) clearly demonstrate presence of the two B chromosomes. Nevertheless, other cell divisions from the same individual show no trace of the chromosomes of that kind (Fig. 2c).
Mitotic divisions in Pnigalio agraules (a–c) and Pnigalio gyamiensis (d–f). a Fragment of a metaphase plate with two B chromosomes b Same individual, late prophase with two B chromosomes c Same individual, prometaphase without B chromosomes d–f Metaphase plates with three, five and six B chromosomes respectively. Arrows indicate B chromosomes. Bar = 10 µm.
Pnigalio gyamiensis Myartseva & Kurashev, 1990 (Figs 1n, 2d–f). The overall karyotype structure as in the preceding species (Table 2), but the second and third chromosome pair can be either metacentric or submetacentric, and the last chromosome pair is subtelocentric (Fig. 1n). In addition, most metaphase plates carry a few very small apparently subtelocentric or acrocentric B chromosomes (usually two to five, but sometimes one or six; Figs 1n, 2d–f).
The family Eulophidae is the most karyotypically studied group of parasitoids of its taxonomic rank in terms of the relative number of studied species. The results presented here provide new information on chromosome number and morphology in certain groups of the family. All species listed in the present paper (except for Pnigalio agraules and Pnigalio gyamiensis; see below) as well as the genera Mischotetrastichus Graham, 1987, Minotetrastichus Kostjukov, 1977 and Chrysocharis Förster, 1856 were studied for the first time. In addition, new karyotypic information was obtained for the genus Cirrospilus Westwood, 1832. Within this genus, only the chromosome number was studied earlier for Cirrospilus diallus Walker, 1838 (n = 6;
Nevertheless, a few deviations from the above mentioned pattern have been recorded up to now, including “Elachertus sp.” with n = 8 (
Chromosomal rearrangements involved in the karyotype evolution of the Eulophidae possibly include chromosomal fusions in a few species (e.g. Minotetrastichus frontalis and Chrysocharis laomedon). In these cases, the smallest chromosome fused to one of the larger elements to form a karyotype with n = 5. On the other hand, an increase in chromosome number in certain groups (e.g. in the genus Hyssopus) could take place by aneuploidy and the subsequent restoration of even chromosome numbers (
Karyotype structure found in Mischotetrastichus and Minotetrastichus showed certain resemblance to that of Tetrastichus Haliday, 1844 s.str. Specifically, metacentrics of the last pair are substantially shorter than the preceding ones (
The meiotic figures obtained in Chrysocharis sp. aff. laomedon and Mischotetrastichus petiolatus generally correspond to mitotic karyotypes of these parasitoids. Specifically, bivalents apparently formed by metacentric chromosomes are relatively large and gradually decrease in size. These ring-like bivalents bear two terminal/subterminal chiasmata in diplotene. Alternatively, the only acrocentric pair found in Mischotetrastichus petiolatus forms a small open bivalent with a single chiasma.
We have also detected B chromosomes in Pnigalio agraules and Pnigalio gyamiensis. Interestingly, chromosome sets of both members of this genus have been recently examined by
The present research also revealed differences between karyotypes of closely related taxa (e.g. within the genus Chrysocharis), thus confirming that chromosomal studies can be used for identifying cryptic species in the Eulophidae, as in many other parasitoid families (