Citation: Adilardi RS, Ojanguren Affilastro AA, Martí DA, Mola LM (2014) Cytogenetic analysis on geographically distant parthenogenetic populations of Tityus trivittatus Kraepelin, 1898 (Scorpiones, Buthidae): karyotype, constitutive heterochromatin and rDNA localization. Comparative Cytogenetics 8(2): 81–92. doi: 10.3897/CompCytogen.v8i2.6461
Tityus trivittatus Kraepelin, 1898 is the most medically important scorpion species of Argentina, and parthenogenetic populations are present in the major cities of this country. We performed a detailed cytogenetic analysis of specimens of three synanthropic parthenogenetic populations, all distant about 900 km from each other, using Ag-NOR, C-banding, DAPI/CMA3 staining and FISH with autologous 28S rDNA probes. The karyotype of females and embryos from the three populations showed 2n=6, with two large and four middle-sized holokinetic chromosomes. Constitutive heterochromatin was found in terminal and interstitial location and its pattern allowed the identification of three chromosome pairs. NORs were found on the terminal heterochromatic region of one pair of middle-sized chromosomes. The use of fluorochromes to characterize heterochromatin showed the absence of GC-rich heterochromatin and a low and variable number of AT-rich heterochromatic regions. We propose that a possible explanation for the lack of karyotypic variation between these geographically distant populations could be a recent colonization of urban areas by human means of synanthropic specimens from a single lineage of northeastern Argentina.
Scorpion, holokinetic chromosomes, parthenogenesis, karyotype, FISH, NOR
Tityus C. L. Koch, 1836 (Buthidae) is the most diversified genus of the order Scorpiones, with about 200 described species. It occurs from Central America to southern South America, in tropical and temperate areas. Several species of this genus are medically important, and most of the dangerous scorpion species in South America belong to the genus Tityus (
Tityus trivittatus Kraepelin, 1898 is the most medically important scorpion species of Argentina and it is responsible for several casualties (
Parthenogenesis in Tityus is quite common and besides Tityus trivittatus it has been mentioned to occur in several species, i.e. Tityus columbianus (Thorell, 1876), Tityus confluens Borelli, 1899, Tityus metuendus Pocock, 1897, Tityus serrulatus Lutz & Mello, 1922, Tityus stigmurus (Thorell, 1876) and Tityus uruguayensis Borelli, 1901 (
In this contribution, we have cytogenetically studied specimens from three synanthropic parthenogenetic Argentinean populations of Tityus trivittatus, from Buenos Aires, Posadas, and Catamarca cities, all distant about 900 km from each other. The karyotype, constitutive heterochromatin distribution and composition, and ribosomal DNA localization were characterized.
We have studied females and embryos of Tityus trivittatus collected from urban populations at the cities of Buenos Aires (34°35.66'S, 58°24.68'W) and Posadas (Misiones province) (27°24.99'S, 55°55.96'W), both in Argentina. Seven females and eleven embryos (of four of these females), were collected by the authors in old subterranean tunnels below the children's Hospital “Dr Ricardo Gutiérrez”, placed in a highly urbanized area of Buenos Aires city. Nine females and seven embryos (of one of these females) were collected by the authors in a backyard of a house in the periphery of Posadas city, Misiones province. Also, two adult females (one of them with six embryos) were provided by the Department of Zoonoses of Catamarca province, Argentina. The exact locality of the specimens of Catamarca is unknown, but these specimens are most likely to have been collected in the city of San Fernando del Valle de Catamarca (28°28.14'S, 65°46.77'W), the biggest city of the province, where the Department of Zoonoses is placed.
All the specimens were carried alive to laboratory and killed by cooling down to -20°C. Their ovaries and embryos were dissected in saline solution (0.154 M NaCl), incubated in hypotonic solution (1:1 saline solution:distilled water) for 30 min, then fixed for 30 min in a freshly prepared Carnoy fixative (ethanol:chloroform:acetic acid, 6:3:1) and stored in fresh fixative. Pieces of ovaries or embryos were placed on slides and dissociated in a drop of 60% acetic acid with tungsten needles. Preparations with a drop of suspension were placed on a heating histological plate at 40–45°C; suspension was spread on the slides using a tungsten needle.
Conventional staining was made with 5% Giemsa solution in distilled water for 12–15 minutes. The C-banding was performed according to the protocol described by
Ribosomal genes were detected by Fluorescence in situ hybridization (FISH) technique with 28S rDNA probe. Total genomic DNA of Tityus trivittatus was extracted using a DNeasy Tissue Kit (QIAGEN, Hilden, Germany). Unlabelled 28S rDNA probes were generated by PCR using primers 28Sa (5´-GACCCGTCTTGAAACACGGA-3´) and 28Sb (5´-TCGGAAGGAACCAGCTACTA-3´) (
To determine the karyotype, chromosome measurements of well-spread prometaphase cells from specimens of each population were made using Micro-Measure software, version 3.3 (
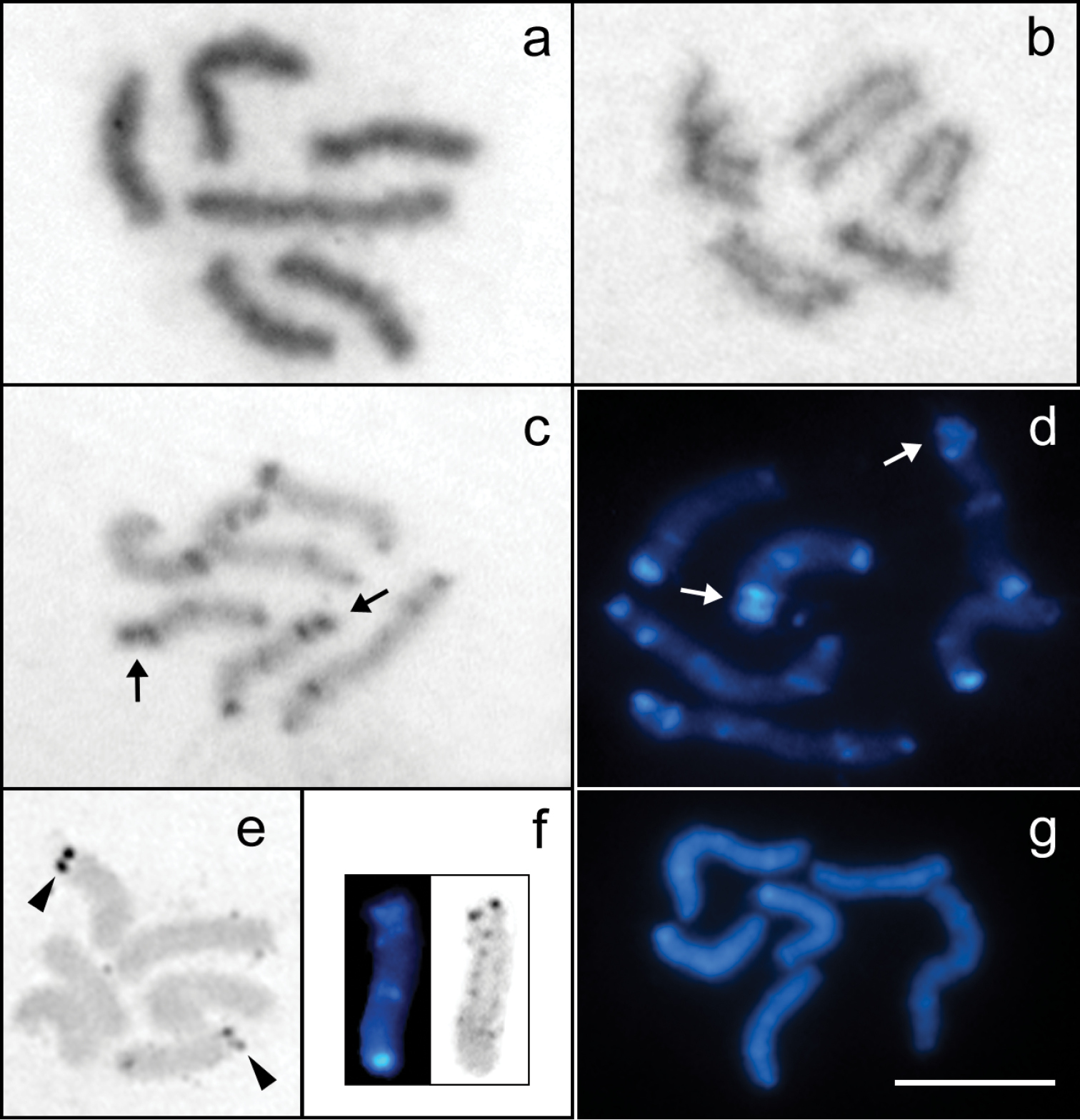
The study of females and embryos of Tityus trivittatus from the parthenogenetic populations of Buenos Aires, Posadas, and Catamarca showed the same chromosome number of 2n=6, with two large and four middle-sized holokinetic chromosomes (Fig. 1a). Each large-sized chromosome presented an average value of 20.72% of the TCL, and the average value of the similar sized medium chromosomes was 14.64% of the TCL (Table 1) (Fig. 2c). The very few cells observed at early anaphase showed parallel arrangement of the sister chromatids, which is characteristic of holokinetic chromosomes (Fig. 1b).
Mitotic cells of Tityus trivittatus (2n=6). a Giemsa-stained prometaphase b Early anaphase c C-banded prometaphase stained with Giemsa (Buenos Aires city) d C-banded prometaphase stained with DAPI (Buenos Aires city) e Silver-stained metaphase f Sequential C-banding and silver staining on chromosome 2 g DAPI-banded prometaphase after DAPI/CMA3 staining. The arrows point to the double-banded terminal region of pair 2. Arrowheads point to the NORs. Scale bar= 10 µm.
Chromosome measurements of the studied populations of Tityus trivittatus. Relative lengths expressed as percentage of total chromosome length (%TCL). Mean values and their standard deviations (SD) are given.
| Chromosome number | Buenos Aires | Catamarca | Posadas |
|---|---|---|---|
| %TCL ± SD | %TCL ± SD | %TCL ± SD | |
| 1 | 21.53 ± 0.99 | 21.54 ± 0.60 | 20.89 ± 0.79 |
| 2 | 19.96 ± 0.34 | 20.45 ± 0.65 | 19.92 ± 0.93 |
| 3 | 15.37 ± 0.57 | 15.44 ± 0.51 | 15.87 ± 0.55 |
| 4 | 14.93 ± 0.34 | 14.82 ± 0.45 | 15.07 ± 0.49 |
| 5 | 14.35 ± 0.33 | 14.28 ± 0.48 | 14.41 ± 0.63 |
| 6 | 13.86 ± 0.40 | 13.47 ± 0.55 | 13.84 ± 0.48 |
The study of specimens from the three localities revealed a complex pattern of C-bands with terminal, subterminal and interstitial localization, which made it possible to identify three chromosome pairs. This pattern was observed both with Giemsa and DAPI staining, although DAPI allowed a better resolution of smaller C-bands. The two large-sized chromosomes (pair 1) presented terminal and subterminal C-bands at each terminal region and one submedial band. The heterochromatic bands at one of the terminal regions are closer and the submedial band is located near of these bands. A pair of middle-sized chromosomes (pair 2) carried a C-band in one terminal region, a medial C-band and a conspicuous terminal and a subterminal C-band at the other terminal region. The other middle-sized chromosomes (pair 3) carried C-bands at each terminal region and a subterminal C-band (Figs 1c, d, 2c). In the specimens from Buenos Aires one of the terminal bands of pair 3 is more conspicuous and the subterminal band is closer to it.
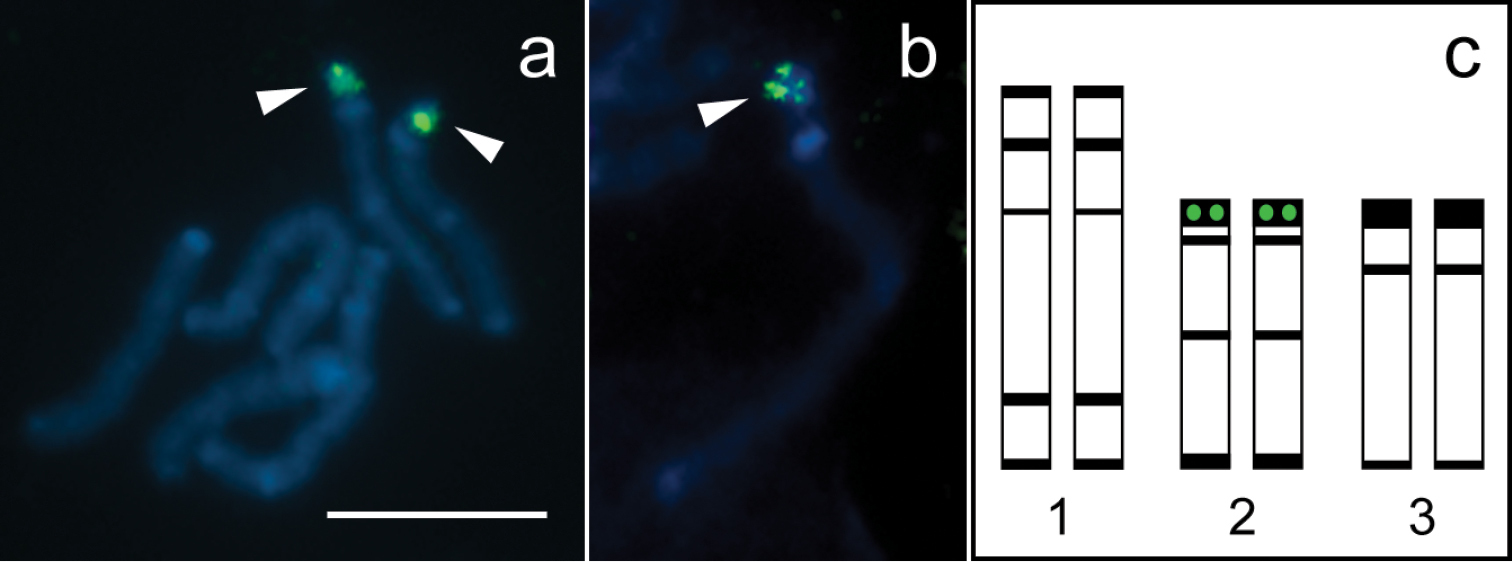
Fluorescence in situ hybridization with 28S rDNA probe and idiogram of the karyotype of Tityus trivittatus. a Mitotic prometaphase with hybridization signals b Chromosome 2 at late prophase with hybridization signal; c. Idiogram showing distribution of constitutive heterochromatin (black bands) and 28S rDNA clusters (green circles). Chromosomes are counterstained with DAPI (blue). Arrowheads point to hybridization signals (green). Scale bar= 10 µm.
Silver staining visualized active NORs at a terminal region of two middle-sized chromosomes (Fig. 1e). Cells with sequential C-banding and silver staining showed that NORs are located at the double-banded terminal region of pair 2 (Fig. 1f).
DAPI/CMA3 sequential staining revealed no bright CMA3 bands. Most cells showed chromosomes homogeneously stained with DAPI. Other cells showed some bright DAPI bands that were coincident with C-bands (Fig. 1g). The number of bright DAPI bands was less than the number of C-bands, and the smaller C-bands were not detected. This technique did not provide reliable results, since the number of DAPI bands was variable between cells with the same degree of chromosome condensation.
DAPI counterstaining in FISH technique revealed a similar pattern of bright bands as C-banding, which allowed for identification of each chromosome pair. Hybridization signals with the autologous 28S rDNA probes were located at the double-banded terminal region of pair 2 (Fig. 2a, c). Late mitotic prophase chromosomes revealed that the rDNA cluster is embedded in the conspicuous terminal C-band of pair 2 (Fig. 2b).
The chromosome number found in the specimens of the Argentinean populations of Tityus trivittatus herein studied is one of the lowest in Buthidae, and it is also present in Tityus martinpaechi Lourenço, 2001 and some individuals of Tityus bahiensis (Perty, 1834) (
In other species of Tityus,
The use of DAPI and CMA3 fluorochromes to characterize heterochromatin of Tityus trivittatus showed the absence of GC-rich heterochromatin and a low and variable number of AT-rich heterochromatic regions, which were coincident with some of the bands revealed by C-banding. In other Buthidae species the number of heterochromatic regions revealed by DAPI/CMA3 staining was also lower than that visualized by C-banding and these regions were almost exclusively AT-rich (only Tityus martinpaechi and Rhopalurus agamemnon (C. L. Koch, 1839) show GC-rich terminal regions in one chromosome pair) (
The number and terminal location of NORs, as well as their association with constitutive heterochromatin found in the specimens of Tityus trivittatus, are all common features reported in other species of Tityus (
Tityus trivittatus is an invasive synanthropic species that easily colonizes urban areas due to its great adaptability, ubiquity and parthenogenetic reproduction. This species was probably introduced into Buenos Aires city during the first half of the twentieth century by anthropogenic means (
Taking into account the high incidence of intra- and interpopulation chromosome rearrangements reported in other species of Tityus, further cytogenetic studies of unequivocally identified sexual and parthenogenetic populations of Tityus trivittatus are needed to reveal potential chromosome variation within this species.
This work was supported by grants from the National Council of Scientific and Technological Research (CONICET) (PIP 0342), National University of Buenos Aires (UBA) (Ex 0859) and ANPCyT (PICT 2010-1665) to Drs L. Poggio and L. Mola and ANPCyT (PICT 2010-1764) to Dr A. A. Ojanguren-Affilastro. The authors wish to thank to Dr Carolina Guilleron and Daniel Hermann from Administración Nacional de Laboratorios e Institutos de Salud “Dr Carlos G. Malbrán” for providing the specimens of Catamarca and to Dr María Isabel Duhau, Dr Valeria Malinovsky, Lic. Daniela Rocco, Eng. Fernando Díaz and Dr Adolfo de Roodt for their help in specimen collection at the “Ricardo Gutiérrez” hospital. We are also grateful to Lic. Elio Castillo for his technical advice and to Drs Alexandra Gottlieb and Marcela Rodriguero for their valuable assistance in obtaining FISH probes.