Citation: Gemi G, Lui RL, Treco FR, Paiz LM, Moresco RM, Margarido VP (2014) Basic cytogenetics and physical mapping of 5S and 18S ribosomal genes in Hoplias malabaricus (Osteichthyes, Characiformes, Erythrinidae) from isolated natural lagoons: a conserved karyomorph along the Iguaçu river basin. Comparative Cytogenetics 8(3): 211–222. doi: 10.3897/CompCytogen.v8i3.7084
Erythrinidae include Neotropical teleost fish that are widely distributed in South America. Hoplias Gill, 1903 include two large groups: H. malabaricus Bloch, 1794 and H. lacerdae Miranda Ribeiro, 1908. Hoplias malabaricus is characterized by remarkable karyotype diversity, with some karyomorphs widely distributed geographically while others are more restricted to certain river basins. Cytogenetic analyzes were performed in a population of Hoplias malabaricus from the Wildlife Refuge of Campos de Palmas, the Iguaçu River basin. The specimens showed diploid number of 42 chromosomes (24m+18sm) without differentiated sex chromosomes system. The impregnation by silver nitrate showed multiple AgNORs. Seven pairs (4, 7, 10, 13, 16, 20 and 21) carrying 18S rDNA were detected by FISH. Heterochromatin was verified in the centromeric and pericentromeric region of most chromosomes and the terminal region of some pairs. FISH with 5S rDNA probes showed two chromosome pairs carrying these sites in the interstitial region (8 and 14). The data obtained in this study are similar to those found for two other populations of H. malabaricus already studied in the basin of the Iguaçu River, confirming the hypothesis that this species is natural, not having been introduced, as well as having an intrinsic characteristic, such as the largest number of sites of 18S rDNA.
Chromosomal conservadorism, double-FISH, evolution, karyotype, rDNA
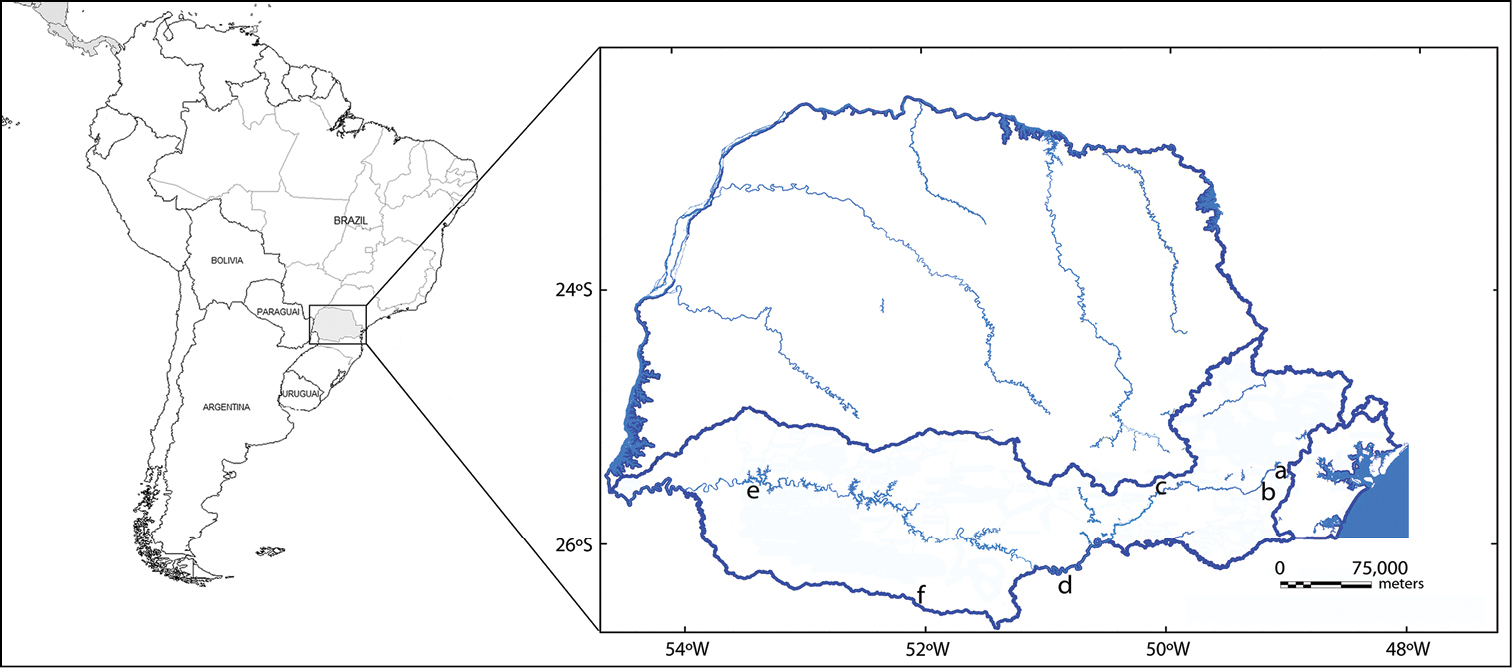
The basin of the Iguaçu River, located in the southern region of the State of Paraná, is comprises a drainage area of 69, 373 square km and a length of 1, 275 km in its main riverbed. Its springs emerge from Serra do Mar and flow towards the First Plateau, or Plateau of Curitiba, and to the Second and Third Plateau. In the latter, the Iguaçu river basin is bordered by the Plateau of Palmas at the border of the State of Santa Catarina (
Erythrinidae are characterized by a sedentary lifestyle which consequently reduces gene flow between the populations that inhabit the same basin since they do not overcome obstacles, such as waterfalls (
According to
With regards to the occurrence of Hoplias malabaricus in the basin of the Iguaçu river, there is a controversy as to its origin in this location. According to
In this sense, the objective of this work was to study - through cytogenetic techniques - a population of Hoplias malabaricus collected in a natural lagoon in the region of Palmas, in the far south of the State of Paraná – Brazil. This lagoon has no contact with other aquatic environments and is isolated from other river systems, in order to better understand the geographical distribution of the group in the basin of the Iguaçu river.
Four specimens were collected (2 males and 2 females) of Hoplias malabaricus from isolated lagoons in the region of Palmas of the Wildlife Refuge of Campos de Palmas, in the Iguaçu river basin, belonging to the State of Paraná – Brazil (Fig. 1). This reduced sample is duet to the collections being made on a conservation unit, and a major sampling would be justified if intra- or interpopulational chromosomal polymorphisms were observed. The samples were anesthetized and sacrificed by an overdose of clove oil (
Map of sampling sites of Hoplias malabaricus populations in Iguaçu river basin: a Piraquara b São José dos Pinhais c Palmeira d Poço Preto e Nova Prata do Iguaçu and f Palmas (present paper).
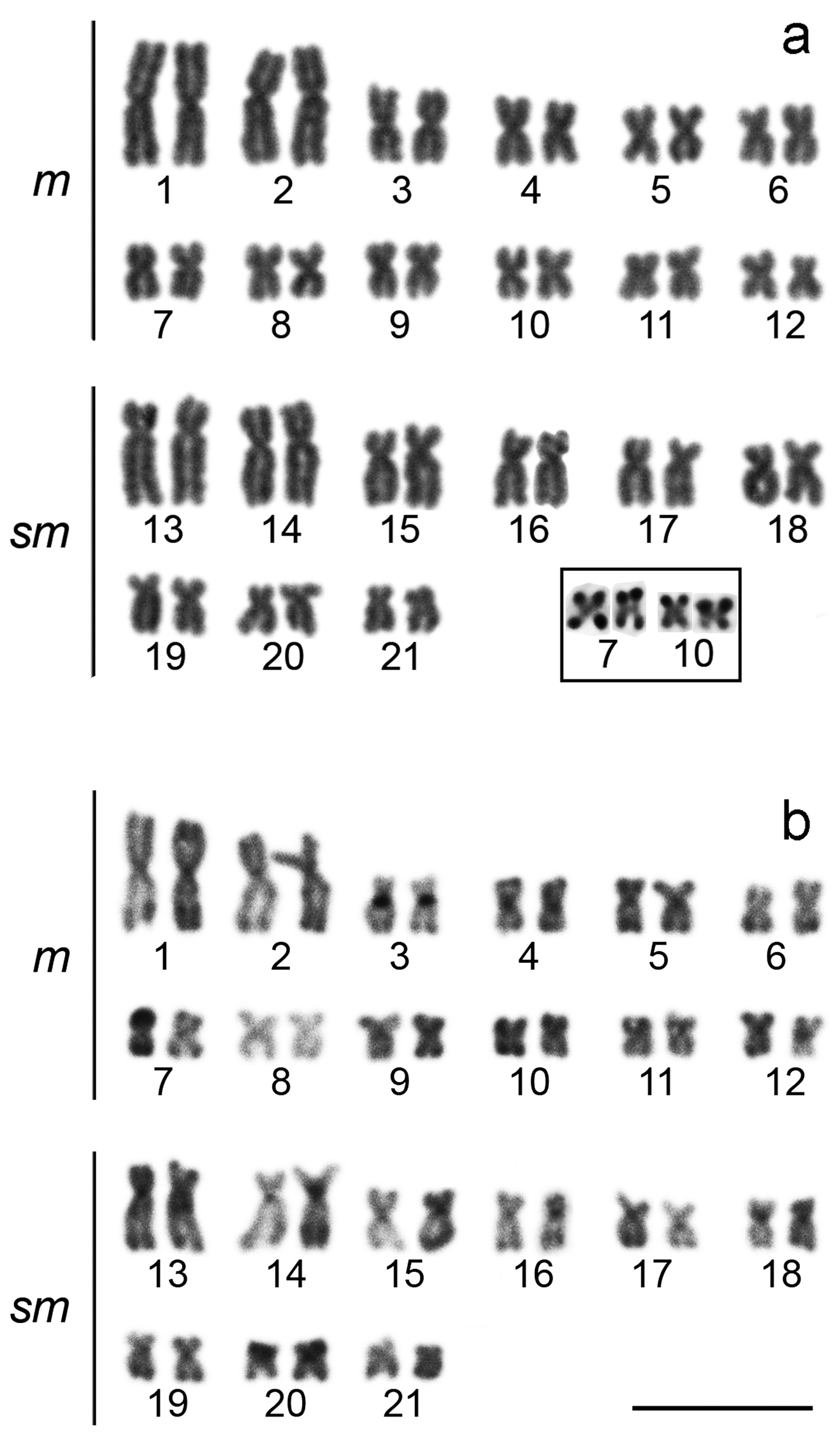
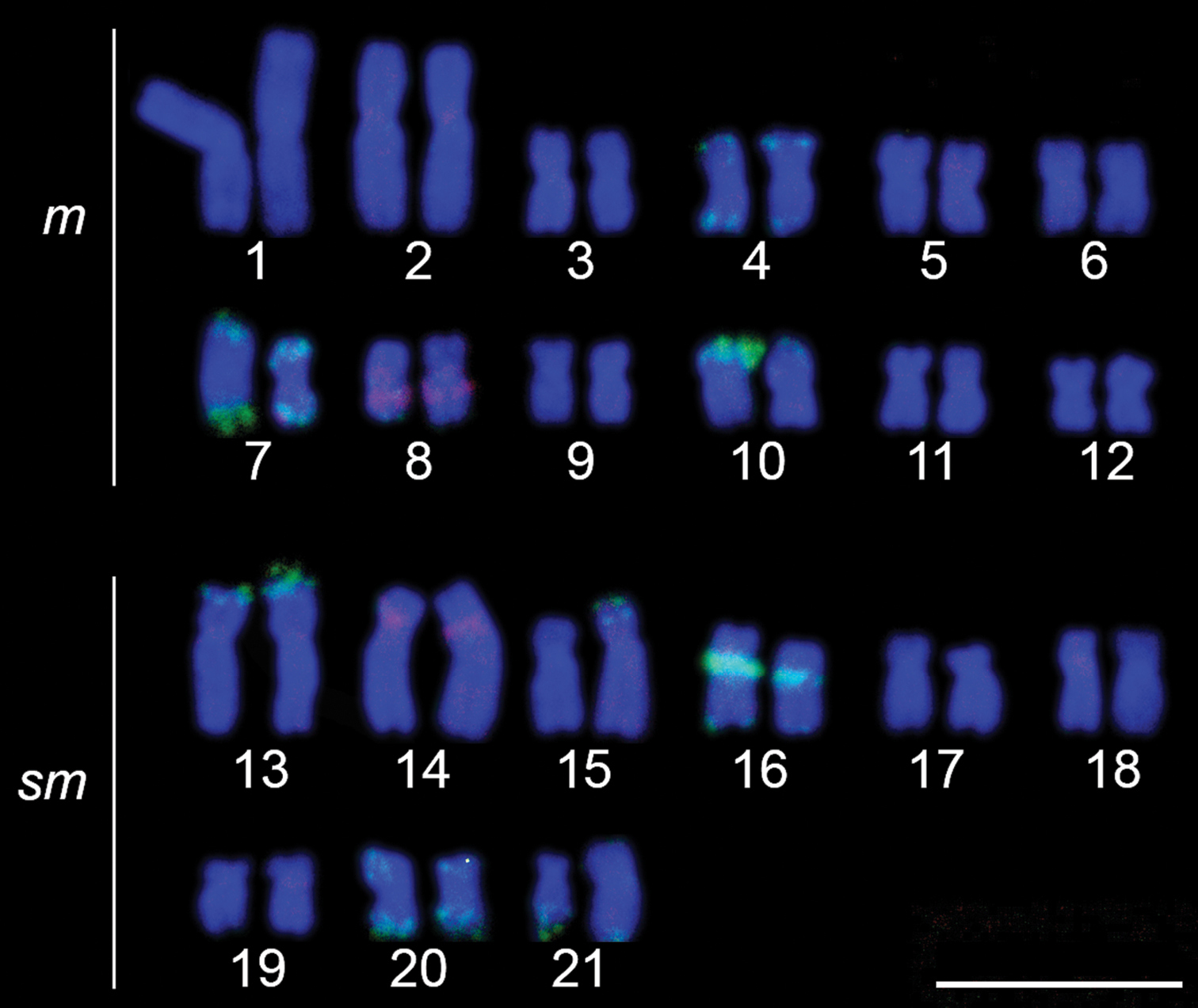
The cytogenetic analysis observed diploid number of 42 chromosomes with 24 metacentric chromosomes and 18 submetacentric chromosomes, for male and female, and without a sex chromosome system (Fig. 2). The impregnation by silver nitrate showed multiple AgNORs, ranging from 4 to 6 NORs. The analyzed metaphases with silver nitrate impregnation presented bi-telomeric labels in the metacentric pair 7 and telomeric labels on the short arm of the metacentric pair 10 (Fig. 2, in box), coinciding with 18S rDNA, evidenced in FISH (Fig. 3). Five other pairs carrying rDNA 18S were marked by FISH, the metacentric 4 in both telomeric regions, the submetacentric pair 13 in the telomeric region of the short arm, pair 16 in the interstitial region of the long arm, pair 20 in both telomeric regions, and pair 21 in the terminal region of the long arm (Fig. 3). The C-banding revealed heterochromatin in the centromeric and pericentromeric region in most chromosomes of the complement, as well as bitelomeric and terminal heterochromatin in some chromosomes, these being coincident with the AgNORs (pairs 7 and 10) (Fig. 2). The FISH with 5S rDNA probe revealed two pairs of chromosomes, being interstitial on the long arm of the metacentric 8 and on the short arm close to the centromere of the submetacentric 14 (Fig. 3).
Karyotypes of Hoplias malabaricus stained with Giemsa (a) and treated through the C-banding (b). The AgNORs bearing chromosome pairs (7 and 10) are presented in box. Bar = 10 µm.
Karyotype of Hoplias malabaricus hybridized with 5S rDNA (digoxigenin, red) and 18S rDNA (FITC, green) probes. Bar = 10 µm.
Hoplias malabaricus comprises a complex of species due to its wide karyotype diversity, and some karyomorphs are geographically widely distributed, while others have lower distribution and are restricted to certain basins, and even sympatric karyomorphs may occur without the detection of hybrids (
For the populations of karyomorph A of Hoplias malabaricus from the Iguaçu river, the karyotype formula does not show any clear marker to differentiate populations throughout this basin (
The distribution of heterochromatin in all the karyomorphs of the Hoplias malabaricus complex has often been described in the terminal and pericentromeric region of some pairs of chromosomes (
The analyses carried out by
Only a population with hybridization data with 18S rDNA is described in literature regarding the Iguaçu river, with four pairs being detected, one pair with bitelomeric marking, one interstitial pair and two pairs with terminal marking. All these pairs of the previous study (
This study showed two pairs of 5S rDNA sites carrying chromosomes. Previous studies showed that this marker varies in number of sites among populations of karyomorph A, with a small metacentric pair with interstitial marking that seems to be conserved (
With the uplift of the Iguaçu Falls, an effective geographic isolation was created for the ichthyofauna of the First and Second Plateaus in the largest part of the Iguaçu river (
Therefore, the population analyzed in this study showed the same diploid number, karyotype formula, lack of a differentiated sex chromosomes system when compared to other populations of the Iguaçu river, in addition to sharing some characteristics with respect to the number and location of AgNORs, distribution of heterochromatin and 18S rDNA sites. These data confirm the hypothesis that Hoplias malabaricus is natural to the Iguaçu River, and in spite of presenting some intrinsic characteristics of this population, it represents the same evolutionary unit along the basin, which is in the process of allopatric differentiation through the setting of small rearrangements in the microstructure.
Cytogenetical data of Hoplias malabaricus populations from Iguaçu river basin.
| Locality | Karyomorph | Karyotype formula | AgNORs | Heterochromatin (C-banding) | 18S rDNA | 5S rDNA | Reference |
|---|---|---|---|---|---|---|---|
| Piraquara municipality (PR) | A | 20m+22sm | Multiple:- 2 to 6 chromosomes (1 bitelomeric pair) | Pericentromeric and interstitial | - | - | 1 |
| São José dos Pinhais municipality (PR) | B | 24m+16sm+2st (XX/XY) | Multiple | Pericentromeric | - | - | 1 |
| Poço Preto municipality (SC) | A | 42 m-sm | - | - | - | - | 2 |
| Palmas municipality (PR) | A | 24m+18sm | Multiple:- pair 7, m, bitel- pair 10, m, tel, sa | Pericentromeric and terminal | - pair 4, m, bitel- pair 7, m, bitel- pair 10, m, tel, sa- pair 13, sm, tel, sa- pair 16, sm, int, la- pair 20, sm, bitel- pair 21, sm, tel, la | - pair 8, m, int, la- pair 14, sm, int, sa | 3 |
| Nova Prata do Iguaçu municipality (PR) | A | 24m+18sm | Multiple:- 3 to 8 chromosomes | Pericentromeric and terminal | - | - | 4 |
| Palmeira municipality (PR) | A | 24m+18sm | Multiple (2 to 7 chromosomes):- pair 10, m, bitel- pair 16, sm, int, la- pair 21, sm, tel, la | Pericentromeric and terminal | - pair 4, m, tel, la- pair 10, m, bitel- pair 16, sm, int, la- pair 21, sm, tel, la | - | 4, 5, 6 |
PR: Paraná state, Brazil; SC: Santa Catarina state, Brazil; m: metacentric; sm: submetacentric; tel: telomeric; bitel: bitelomeric; int: interstitial; la: long arm; sa: short arm.References: 1 -
The authors are grateful to Dr. Weferson Junio da Graça for the identification of the specimens and to Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for authorizing the capture of the fishes (License number: SISBIO 10522-1). This study was financed by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), by Fundação Araucária (Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).